Left ventricular assist device therapy: the Kuwait experience
Introduction
Heart failure is an epidemic with a current prevalence of over 5.8 million patients in the USA and close to 23 million patients worldwide. The disease carries a high mortality risk of greater than 50% at 5 years (1). Heart transplantation, the “Gold Standard” for treatment of end-stage heart disease, is not viable in Kuwait due to the severe shortage of donor organs and increasing number of heart failure patients. Over the last 2 years left ventricular assist device therapy (LVAD) has emerged as a life-saving treatment for crash and burn patients either as a bridge to transplantation (BT) or destination therapy (DT) (2-6). Although current studies reveal 1- and 2-year post-LVAD implantation survival rates of 80% and 70% respectively, the outcome is usually better in stable patients (7).
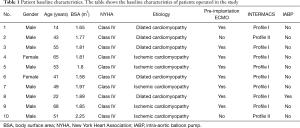
Between January 2015 and October 2017, ten patients (eight males and two females) with a mean age of 48 years (range, 14–68 years) received LVAD as a bridge to BT or DT. All patients were in New York Heart Association (NYHA) class IV. Four patients were diagnosed with dilated cardiomyopathy and six patients with ischemic cardiomyopathy. The mean ejection fraction (EF) was 15% (range, 10–25%). Eight patients were classified according to the Interagency Registry of Mechanically Assisted Circulatory Support (INTERMACS) as profile 1, supported by preoperative arterio-venous (AV) extracorporeal membrane oxygenator (ECMO) for 2–4 days before implantation of the device and two patients were INTERMACS profile 2 (8,9). Three patients received HeartMate II and seven patients received HeartWare devices. In this paper we describe the first clinical experience with LVAD therapy of heart failure patients at our clinic.
Methods
Patient demographics
This study was reviewed and approved by the research ethics board of Al Adan Hospital, Kuwait. We reviewed the clinical records of ten consecutive patients who underwent LVAD implantation at Al Dabbous Cardiac Center in Kuwait between the period January 2015 and October 2017. We collected detailed information on these selected patients, including demographical characteristics, preoperative ECMO support, concomitant valve lesions and procedures done during device implantation. The aim of the study was to assess 30-day mortality rates of patients following LVAD implantation.
Surgical technique
The procedure was undertaken through a conventional median sternotomy except in one patient were the LVAD was implanted with a minimally invasive technique (L 5th interspace thoracotomy and upper mini-sternotomy 2nd ICS) (4,10). Standard cardiopulmonary bypass (CPB) with central aortic cannulation and right atrial cannulation without fibrillatory or cardioplegic arrest except in two patients: one patient needed coronary artery grafting to a large posterior descending artery (PDA) and another patient needed aortic valve repair for grade III aortic regurgitation. The apical sewing cuff of the inflow cannula was placed using interrupted 2-0 Ticron sutures. A side biting aortic clamp was placed on the ascending aorta to suture the outflow graft. Three patients needed tricuspid valve repair, and this was done on a beating heart as the pump was being primed. The electrical driveline was tunneled thru the abdominal wall. The LVAD was desired and LVAD support started. Before leaving the operating room complete reversal of anticoagulation was performed. The ECMO was explanted following the LVAD implantation in all patients except 2. In one patient it was kept for postoperative support of the right ventricle after it was converted to femoral vein-pulmonary artery. In the second patient it was kept as AV ECMO for oxygenation support as the patient has had massive bilateral pulmonary hemorrhage and hemoptysis 1 day after the ECMO was placed pre-operatively. All operations were performed by a single surgeon (R Tarazi, MD).
Results
Ten NYHA class IV patients with a mean age of 48 years and 10–25% EF were admitted to our clinic for dilated cardiomyopathy and ischemic cardiomyopathy. All patients’ basic characteristics are shown in Table 1. The patients received LVAD as a bridge to BT or DT. Eight patients were classified as INTERMACS 1 and received preoperative AV ECMO for 2–4 days prior to LVAD implantation and two patients were classified as INTERMACS profile 2 (8,9). Three patients were implanted with HeartMate II and seven patients received HeartWare devices. The surgical data and operative outcomes as well as the survival of patients are summarized in Tables 2,3.
Full table

Full table

Full table
Discussion
Early experience in Kuwait with LVAD therapy as a life-saving measure for “Crash and Burn” patients was excellent with a 30-day mortality of 0% and no major surgical complications or LVAD thrombosis. This may have been due to the early institution of AV ECMO and the availability of resources in our center. Postoperative anticoagulation was performed as per our institutional policy. After the chest tube drainage is minimal heparin is started to maintain an activated clotting time (ACT) of 180 seconds. After removal of the chest tubes +/− extubation warfarin is started with a target International Normalized Ratio (INR) of 2.0–2.5 and one plavix 75-mg tablet every week (11). In spite of our aggressive anticoagulation we didn’t face any bleeding complications or in-hospital tamponade except in one patient who had heparin induced thrombocytopenia and underwent LVAD placement with Angiomax anticoagulation. This patient bled massively in the operating room but eventually stopped bleeding and the chest was closed.
Survival rate reported in the literature ranges between 20–30% among patients who were weaned after receiving AV ECMO for cardiac arrest or cardiogenic shock. Much better outcomes are seen if ECMO is provided after which the patient if he is a candidate receives a long-term LVAD as a BT or DT (12). We believe that ECMO should be inserted as early as possible to minimize end organ damage and define further therapy. Shock II study showed that in patients with cardiogenic shock, there is no effect of intra-aortic balloon pump (IABP) treatment on microvascular perfusion and we rarely use it (13).
Currently, consensus is to intervene on the aortic valve at the time of LVAD implant if the patient has moderate or severe aortic insufficiency (AI) (class I, Level of Evidence C) (14). The aortic valve can be either be repaired or replaced with a bioprosthetic valve. The central coaptation repair stitch (Park’s stitch) has been shown to be effective and durable in reducing the AI as well as improving survival. The opening of the aortic valve is maintained to allow ejection, without the additional cross-clamp time that would be required for valve replacement, and could be detrimental for right ventricular function (15,16).
Brewer et al. included 101 patients in whom concomitant tricuspid valve repair was performed with LVAD implantation in patients with moderate or severe tricuspid regurgitation (17). In his series tricuspid valve repair was associated with improved survival and trends towards less right ventricular failure. Three of our patients underwent concomitant tricuspid valve repair on a beating heart using the modified De-Vega procedure to treat tricuspid regurgitation (17,18).
Contrary to available data, we have been implanting LVAD’s in patients with INTERMACS 1 with very good results. In the last patient the LVAD was placed minimally invasively and despite moderate right ventricular dysfunction preoperatively the patient did not need ECMO for right ventricular support and had a rapid recovery. Cowger et al. in an INTERMACS analysis concluded that worst survival as noted in very low volume LVAD centers (≤10 implants/year) (19).
The aim of LVAD implantation in our institution was directed to DT as well as a BT. Two of 4 patients have been transplanted in USA. One is now on the waiting list for transplant in India. The rest of the patients who survived due to their co-morbidities and unavailability of cardiac transplantation are on LVAD as DT.
Conclusions
Our early results of LVAD implantation in Kuwait prove that it is feasible to establish a de novo LVAD program in a low volume cardiac center (Approx. 175 pumps/year) and a low volume LVAD center (≤10 implants/year) with excellent results and 0% 30-day mortality. We believe that the early institution of AV ECMO and the availability of resources in our center made this possible. This has opened a new era of hope for patients who would have died in the past.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was reviewed and approved by the research ethics board of Al Adan Hospital, Kuwait (No. 123888).
References
- Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation 2010;121:948-54. [Crossref] [PubMed]
- Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435-43. [Crossref] [PubMed]
- Schmitto JD, Zimpfer D, Fiane AE, et al. Long-term support of patients receiving a left ventricular assist device for advanced heart failure: a follow-up analysis of the Registry to Evaluate the HeartWare Left Ventricular Assist System. Eur J Cardiothorac Surg 2016;50:834-8. [Crossref] [PubMed]
- Hanke JS, Rojas SV, Avsar M, et al. Minimally-invasive LVAD Implantation: State of the Art. Curr Cardiol Rev 2015;11:246-51. [Crossref] [PubMed]
- Schmitto JD, Hanke JS, Rojas SV, et al. First implantation in man of a new magnetically levitated left ventricular assist device (HeartMate III). J Heart Lung Transplant 2015;34:858-60. [Crossref] [PubMed]
- Schmitto JD, Rojas SV, Haverich A. Left Ventricular Assist Devices for Advanced Heart Failure. N Engl J Med 2017;376:1894. [PubMed]
- Gustafsson F, Rogers JG. Left ventricular assist device therapy in advanced heart failure: patient selection and outcomes. Eur J Heart Fail 2017;19:595-602. [Crossref] [PubMed]
- Stein ML, Robbins R, Sabati AA, et al. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS)-defined morbidity and mortality associated with pediatric ventricular assist device support at a single US center: the Stanford experience. Circ Heart Fail 2010;3:682-8. [Crossref] [PubMed]
- Miller MA, Ulisney K, Baldwin JT. INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support): a new paradigm for translating registry data into clinical practice. J Am Coll Cardiol 2010;56:738-40. [Crossref] [PubMed]
- Schmitto JD, Mokashi SA, Cohn LH. Past, present, and future of minimally invasive mitral valve surgery. J Heart Valve Dis 2011;20:493-8. [PubMed]
- Tsubota H, Ribeiro RVP, Billia F, et al. Left ventricular assist device exchange: the Toronto General Hospital experience. Can J Surg 2017;60:253-9. [Crossref] [PubMed]
- Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant 2015;34:1495-504. [Crossref] [PubMed]
- Jung C, Fuernau G, de Waha S, et al. Intraaortic balloon counterpulsation and microcirculation in cardiogenic shock complicating myocardial infarction: an IABP-SHOCK II substudy. Clin Res Cardiol 2015;104:679-87. [Crossref] [PubMed]
- Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant 2013;32:157-87. [Crossref] [PubMed]
- McKellar SH, Deo S, Daly RC, et al. Durability of central aortic valve closure in patients with continuous flow left ventricular assist devices. J Thorac Cardiovasc Surg 2014;147:344-8. [Crossref] [PubMed]
- Park SJ, Liao KK, Segurola R, et al. Management of aortic insufficiency in patients with left ventricular assist devices: a simple coaptation stitch method (Park’s stitch). J Thorac Cardiovasc Surg 2004;127:264-6. [Crossref] [PubMed]
- Brewer RJ, Cabrera R, El-Atrache M, et al. Relationship of tricuspid repair at the time of left ventricular assist device implantation and survival. Int J Artif Organs 2014;37:834-8. [Crossref] [PubMed]
- Westaby S.. Tricuspid regurgitation in left ventricular assist device patients. Eur J Cardiothorac Surg 2012;41:217-8. [Crossref] [PubMed]
- Cowger JA, Stulak JM, Shah P, et al. Impact of Center Left Ventricular Assist Device Volume on Outcomes After Implantation: An INTERMACS Analysis. JACC Heart Fail 2017;5:691-9. [Crossref] [PubMed]


