Ultrathin strut biodegradable-polymer sirolimus-eluting stents: being wary or going with the flow?
In 2017, the cardiology community celebrated the 40th anniversary of the first coronary angioplasty by Grüntzig in Zurich, Switzerland (1). During this period of time, percutaneous coronary intervention (PCI) rapidly progressed from plain balloon angioplasty to bare-metal and drug-eluting stent (DES) platforms, the latter being a first-line therapy for patients presenting with anatomically suitable obstructive disease of coronary arteries (2).
The DES-specific efficacy and safety profile relies on the various combinations of supportive backbones, polymer coatings and antiproliferative drugs. In the last decade, investigations of DES technologies with different polymers and active drugs have attracted considerable interest (3). Amongst others, the evidence of serious adverse events associated with durable polymers belonging to early-generation DES (4) pushed for platforms with more biocompatible durable or completely biodegradable polymers. These coatings aimed at modulating drug-release kinetics through temporary interaction with the vasculature and complete dissolution once the intended function has passed (5). Biodegradable-polymer DES showed improved healing and lower thrombogenicity compared to early-generation durable-polymer DES (6). However, the favorable vascular behavior of biodegradable-polymer DES was not confirmed in comparisons against more biocompatible durable-polymer DES, challenging the role of biodegradable-polymer DES technology in contemporary practice (7). Remarkably, biodegradable-polymer DES technology recently evolved towards ultrathin metallic backbones (≤80 µm of strut thickness) where several new platforms have emerged on the market (Table 1).
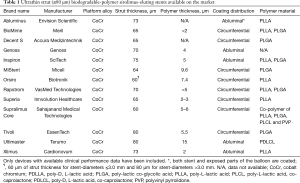
Full table
In light of these considerations, the results of the BIOTRONIK—A Prospective Randomized Multicenter Study to Assess the Safety and Effectiveness of the Orsiro Sirolimus Eluting Coronary Stent System in the Treatment of Subjects With up to Three De Novo or Restenotic Coronary Artery Lesions-V (BIOFLOW V) trial published in Lancet in October 2017 (8) have to be highlighted. The BIOFLOW V study was a multicentre trial which randomly assigned 1,334 patients with a 2:1 ratio to either the ultrathin strut biodegradable-polymer sirolimus-eluting stent (SES, Orsiro, Biotronik, Bülach, Switzerland) or the thin strut fluoropolymer-based everolimus-eluting stent (EES, Xience, Abbott Vascular, Santa Clara, CA, USA). The fluoropolymer-based EES (81 µm of strut thickness) represents the current benchmark durable-polymer DES (3), whilst the investigational device studied in this trial consists of a latest-generation DES with a cobalt-chromium scaffold (60 µm of strut thickness for stent-diameters up to 3.0 mm and 80 µm for stent-diameters >3.0 mm) and a fully biodegradable dual-polymer coating (9). This carrier is composed of a thin-layer of amorphous, hydrogen-rich, silicon carbide, which is in contact with the stent surface, and an asymmetric circumferential layer containing a matrix of poly-L lactic acid (PLLA) loaded with the antiproliferative drug sirolimus (1.4 µg/mm2 of stent surface).
The BIOFLOW trial enrolled a moderately complex population of PCI-patients to support the approval of the ultrathin strut biodegradable-polymer SES from US regulatory agencies. The primary endpoint was target lesion failure [TLF, including cardiovascular death, myocardial infarction (MI)-related to the target vessel, and ischemia-driven target lesion revascularization] 12 months after successful percutaneous revascularization. Although powered to test the hypothesis of non-inferiority for TLF with a Bayesian analysis, the trial displayed the superiority of biodegradable-polymer SES versus fluoropolymer-based EES for the primary endpoint (6% vs. 10%, P=0.047). This significant difference was attributable to a lower incidence of MI related to the target vessel in patients receiving the investigational device as compared to those who not (5% vs. 8%, P=0.039). Interestingly, between 1 and 12 months after PCI, the biodegradable-polymer SES was associated with significantly less stent thrombosis (ST) as compared to fluoropolymer-based EES (0.1% vs. 0.9%, P=0.015). There was no difference with respect to other outcomes. Although the authors should be congratulated for completing such a large-scale randomized trial in a timely fashion, the claims for clinical superiority of biodegradable-polymer SES over fluoropolymer-based EES should be toned down for a couple of reasons.
Firstly, the BIOFLOW V trial reported a 100% posterior probability for ultrathin strut bioresorbable-polymer SES of being non-inferior to fluoropolymer-based EES. A Bayesian approach was chosen to test the hypothesis of non-inferiority. To increase the efficiency in estimation of the performance of devices under investigation, the data from the BIOFLOW V trial were merged with two previous smaller but similarly designed randomized trials [namely, BIOFLOW II (10) and BIOFLOW IV (11) trials]. Notably, the US regulatory agencies have recently recognized the validity of this statistical methodology for comparative studies of medical devices, which potentially leads to smaller trials through a better usage of pre-existing high-quality evidence (12,13). However, a prerequisite to apply the Bayesian analytical method is that historical data are sufficient alike to be pooled (14). In this specific case, the analysis of non-inferiority with re-adjudication of clinical endpoints was restricted to subjects included in the BIOFLOW II and IV trials that fulfilled all BIOFLOW V eligibility criteria. Whether this statistical approach affects the distribution of baseline features among patients included in the original trials and selected for current analyses remains uncertain.
Secondly, the reduction of MI related to the target vessel observed with ultrathin strut biodegradable-polymer SES in the BIOFLOW V trial emerged during the peri-procedural period and persisted at 12-month follow-up. The PLLA of the biodegradable-polymer SES has the slowest degradation kinetic among biodegradable polymers DESs (after 12 to 24 months) (9): for this reason, the lower incidence of MI at 12-month follow-up with biodegradable-polymer SES may not be attributable to the resorption process of the carrier. This is not a novelty (15) and preclinical studies (16,17) found biodegradable-polymer SES associated with low thrombogenicity and accelerated endothelialisation, owing to the backbone design and the highly biocompatible coating as well. Notwithstanding this, whether the unique coating formulation of biodegradable-polymer SES, which aims at reducing the inflammation of surrounding vasculature due to metal components, may be responsible for the lower risk of MI related to the target vessel has yet to be demonstrated.
Thirdly, the BIOFLOW V trial showed a lower risk of late ST with the biodegradable-polymer SES as compared to fluoropolymer-based EES. This observation should be interpreted with caution, since the number of thrombotic events was extremely low and the trial was largely underpowered with respect to this outcome. Noteworthy, the biodegradable-polymer SES investigated in the BIOFLOW V trial has the thinnest struts among contemporary DESs (9). Thin-struts stents were found to accelerate endothelial recovery and re-endothelialization in comparison with thick-struts platforms (18). These mechanical features were likely responsible for the lower risk of ST observed in a recent randomized trial comparing thin-strut versus thick-strut biodegradable-polymer DESs in moderately complex coronary lesions (19). However, imaging data (20) and preclinical models (16) support a highly favourable healing pattern for fluoropolymer-based EES as compared to biodegradable-polymer DESs either with thick- or thin-strut backbone designs. In line with these considerations, whether in the era of biocompatible durable-polymer DESs the peculiar mechanical features of biodegradable-polymer SES contribute to a lower thrombotic risk require further investigation and long-term follow-up data.
Finally, the BIOFLOW V trial lends support to a device-specific healing process among biodegradable-polymer DES platforms (21) and leaves room for studies focused on the performance of biodegradable-polymer SES in high-risk subgroup of patients. For example, any potential benefit of this platform in terms of MI risk would be of certain relevance in patients with ST-segment elevated MI given the high inflammatory vascular milieu. However, the BIOFLOW V trial excluded individuals admitted with this clinical presentation and the potential superiority of biodegradable-polymer SES over fluoropolymer-based EES in this setting remains subject to ongoing investigation (12). Similarly, a recent randomized trial of patients presenting complete chronic occlusions of coronary vessels found inferior angiographic performance with biodegradable-polymer SES compared to fluoropolymer-based EES (22). Disappointingly, the presence of a chronic total occlusion was among the main angiographic exclusion criteria of the BIOFLOW V trial, precluding any further evaluation of biodegradable-polymer SES in this subgroup of complex lesions.
In conclusion, although the Bayesian methodology might represent a supportive statistical tool to facilitate the demonstration of efficacy in future comparative studies of medical devices, the inclusion of patients presenting high-risk clinical and angiographic features remains a goal to be pursued in clinical investigations of contemporary DES platforms. Only this approach will certainly help to disclose any advantage of biodegradable-polymer SES as compared to the best-in-class fluoropolymer-based EES.
Acknowledgements
None.
Footnote
Conflicts of Interest: S Cassese and AL Lahmann have no conflicts of interest to declare. M Joner reports personal fees from Orbus Neich, grants and personal fees from Biotronik, personal fees from Coramaze, personal fees from Astra Zeneca, personal fees from Bristol-Myers-Squibb, outside the submitted work.
References
- Gruntzig A. Transluminal dilatation of coronary-artery stenosis. Lancet 1978;1:263. [Crossref] [PubMed]
- Authors/Task Force m, Windecker S, Kolh P, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541-619.
- Byrne RA, Stone GW, Ormiston J, et al. Coronary balloon angioplasty, stents, and scaffolds. Lancet 2017;390:781-92. [Crossref] [PubMed]
- Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 2006;48:193-202. [Crossref] [PubMed]
- Byrne RA, Joner M, Kastrati A. Polymer coatings and delayed arterial healing following drug-eluting stent implantation. Minerva Cardioangiol 2009;57:567-84. [PubMed]
- Stefanini GG, Byrne RA, Serruys PW, et al. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J 2012;33:1214-22. [Crossref] [PubMed]
- Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Clinical outcomes with bioabsorbable polymer- versus durable polymer-based drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol 2014;63:299-307. [Crossref] [PubMed]
- Kandzari DE, Mauri L, Koolen JJ, et al. Ultrathin, bioresorbable polymer sirolimus-eluting stents versus thin, durable polymer everolimus-eluting stents in patients undergoing coronary revascularisation (BIOFLOW V): a randomised trial. Lancet 2017;390:1843-52. [Crossref] [PubMed]
- Iglesias JF, Roffi M, Degrauwe S, et al. Orsiro cobalt-chromium sirolimus-eluting stent: present and future perspectives. Expert Rev Med Devices 2017;14:773-88. [Crossref] [PubMed]
- Windecker S, Haude M, Neumann FJ, et al. Comparison of a novel biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent: results of the randomized BIOFLOW-II trial. Circ Cardiovasc Interv 2015;8:e001441. [Crossref] [PubMed]
- Saito S. 3rd Generation Sirolimus Eluting Coronary Stent Orsiro. Oral presentation at 81st Japan Circulation Society Congress 2017, Kanazawa, Japan.
- Iglesias JF, Muller O, Zaugg S, et al. A comparison of an ultrathin strut biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent for patients with acute stsegment elevation myocardial infarction undergoing primary percutaneous coronary intervention: rationale and design of the BIOSTEMI trial. EuroIntervention 2017. [Epub ahead of print]. [PubMed]
- Doros G, Massaro JM, Kandzari DE, et al. Rationale of a novel study design for the BIOFLOW V study, a prospective, randomized multicenter study to assess the safety and efficacy of the Orsiro sirolimus-eluting coronary stent system using a Bayesian approach. Am Heart J 2017;193:35-45. [Crossref] [PubMed]
- Gupta SK. Use of Bayesian statistics in drug development: Advantages and challenges. Int J Appl Basic Med Res 2012;2:3-6. [Crossref] [PubMed]
- Pilgrim T, Piccolo R, Heg D, et al. Biodegradable polymer sirolimus-eluting stents versus durable polymer everolimus-eluting stents for primary percutaneous coronary revascularisation of acute myocardial infarction. EuroIntervention 2016;12:e1343-e1354. [Crossref] [PubMed]
- Otsuka F, Cheng Q, Yahagi K, et al. Acute Thrombogenicity of a Durable Polymer Everolimus-Eluting Stent Relative to Contemporary Drug-Eluting Stents With Biodegradable Polymer Coatings Assessed Ex Vivo in a Swine Shunt Model. JACC Cardiovasc Interv 2015;8:1248-60. [Crossref] [PubMed]
- Secco GG, Mattesini A, Fattori R, et al. Time-related changes in neointimal tissue coverage of a novel Sirolimus eluting stent: Serial observations with optical coherence tomography. Cardiovasc Revasc Med 2016;17:38-43. [Crossref] [PubMed]
- Kolandaivelu K, Swaminathan R, Gibson WJ, et al. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation 2011;123:1400-9. [Crossref] [PubMed]
- Jensen LO, Thayssen P, Maeng M, et al. Randomized Comparison of a Biodegradable Polymer Ultrathin Strut Sirolimus-Eluting Stent With a Biodegradable Polymer Biolimus-Eluting Stent in Patients Treated With Percutaneous Coronary Intervention: The SORT OUT VII Trial. Circ Cardiovasc Interv 2016;9:e003610. [Crossref] [PubMed]
- Cassese S, Xhepa E, Ndrepepa G, et al. Vascular response to percutaneous coronary intervention with biodegradable-polymer vs. new-generation durable-polymer drug-eluting stents: a meta-analysis of optical coherence tomography imaging trials. Eur Heart J Cardiovasc Imaging 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Rizas KD, Mehilli J. Stent Polymers: Do They Make a Difference? Circ Cardiovasc Interv 2016;9:e002943. [Crossref] [PubMed]
- Teeuwen K, van der Schaaf RJ, Adriaenssens T, et al. Randomized Multicenter Trial Investigating Angiographic Outcomes of Hybrid Sirolimus-Eluting Stents With Biodegradable Polymer Compared With Everolimus-Eluting Stents With Durable Polymer in Chronic Total Occlusions: The PRISON IV Trial. JACC Cardiovasc Interv 2017;10:133-43. [Crossref] [PubMed]


