Assessment of programmed cell death ligand-1 expression with multiple immunohistochemistry antibody clones in non-small cell lung cancer
Introduction
Programmed cell death-1 (PD-1) and programmed cell death ligand-1 (PD-L1) have been identified as novel targets for immunotherapy in diverse cancer types, including non-small cell lung cancer (NSCLC). Over the last 2 years, the U.S. Food and Drug Administration (FDA) have approved three PD-1 monoclonal antibodies, nivolumab, pembrolizumab and atezolizumab for advanced NSCLCs (1). Most recently, durvalumab has gained approval by FDA to treat patients with locally advanced or metastatic urothelial carcinoma (2).
Although clinical trials have demonstrated unequivocal benefits from this family of drugs, a predictive biomarker is not yet clarified. Since most clinical trials indicate that anti-PD-1/PD-L1 inhibitors have become a beneficial therapy for patients with high PD-L1 expression (3,4), assessing PD-L1 status by immunohistochemistry (IHC) is deemed as a predictive diagnostic test to identify potential responders (5,6). To date, trials have utilized different primary antibody clones and cut-off values for determining the positive staining. These various antibody clones include Dako22C3, Dako28-8, VentanaSP142, and VentanaSP263 that accompany the diagnostic assays for pembrolizumab, nivolumab, atezolizumab, and durvalumab, respectively. However, some studies indicated a substantial rate of response in tumors lacking PD-L1 (7). Furthermore, the prognostic value of PD-L1 expression remains controversial, with some studies showing that patients with high PD-L1 expression exhibit poor prognosis (3,8,9), while others found no prognostic value of PD-L1 expression (10).
The contrasting conclusions do not imply that PD-L1 is of limited value for prognostic and predictive analysis; instead, they might be potentially attributed to the challenging conditions of the PD-L1 assay. Multiple assays can be used to detect the PD-L1 status, including IHC, quantitative immunofluorescence (QIF), and in-situ hybridization (ISH). The latter two are not widely adopted in clinical practice owing to the complicated operation, prolonged duration and high cost. IHC tests allow localizing the targets and observing quantitatively; moreover, these methods are relatively inexpensive, performed rapidly, and accessible widely. Nevertheless, each therapeutic trial used its companions and antibodies without reference to a common standard, thereby leading to varied conclusions, which might result in the different treatment decision. Besides, it might be challenging to evaluate the accuracy and reliability of relevant clinical trials on the basis of varied IHC assays and analytical procedures.
The clinicopathological identification of patients with NSCLC expressing PD-L1 will be highly significant since PD-1 axis inhibitors have been recognized as a vital treatment option for NSCLCs. However, these efforts have been uncoordinated: some studies showed that PD-L1 positivity was significantly associated with male gender, smoking, advanced stage, squamous cell carcinoma, and wild-type epidermal growth factor receptor (EGFR) gene mutation status (11), while others demonstrated minimal association between PD-L1 expression and clinicopathological factors (12,13). Whether the utilization of various antibodies led to these conflicting results is in need to be substantiated.
In the present translational study, we conducted a head-to-head comparison of three primary IHC antibodies directed towards PD-L1, including SP142, SP263 and UMAB228 (an antibody newly developed by Beijing OriGene Technologies). The analytical comparison shed light on the convoluted status of PD-L1 IHC and on the decision making for the treatment of patients.
Methods
NSCLC sample cohort
A study cohort was constructed by reviewing the archives of NSCLC cases at the Department of Pathology, West China Hospital, from 2011 to 2016, retrospectively. Among the 84 cases, 42 resection specimens and 42 biopsies were included. All patients did not receive treatment before surgery or biopsy, including chemotherapy, radiotherapy or immunotherapy; nevertheless, these patients were selected as representative NSCLC cases for the estimation of the expression using multiple antibodies and assessment of the association between PD-L1 and clinicopathological characteristics. The clinicopathological features, including the age of sampling, sex, smoking history, histology, lymph node metastasis status, distant metastasis status, and driven gene mutation status were examined.
According to the ethical requirement, the study was approved by West China Hospital of Sichuan University Biomedical Research Ethics Committee, with the number of the approval 20170426. Besides, participants involved in the study had given informed consent before taking part in.
PD-L1 antibody clones
The tumor tissues were fixed with formalin, embedded in paraffin, and cut into 4 µm-thick sections for IHC staining. Consecutive sections were used to reduce the variability between assays due to tumor heterogeneity. The information of the three commercially available antibodies utilized in this study was summarized in Table 1.
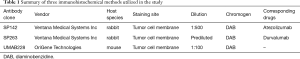
Full table
IHC
Fresh sections were deparaffinized and IHC staining was performed according to the manufacturer’s description. Control slides were stained in parallel to the experimental slides for reproducibility. After the inhibition of the endogenous peroxidase activity for 30 min with 3% H2O2 in methanol, tris-EDTA buffer (pH =8.0) was used for antigen retrieval at 100 °C for 10 min, followed by incubation with primary antibody (Ab) overnight at 4 °C in a humidified chamber. Subsequently, the slides were incubated with goat anti-mouse or goat anti-rabbit (corresponding to the primary Ab), Elivison amplification reagent for 30 min at room temperature, followed by incubation with diaminobenzidine (DAB) for 5 min at room temperature. The slides were then counterstained for 7 min at room temperature with hematoxylin and dehydrated for 1 min using gradient ethanol washes (70%, 85%, 95% and 100%) and finally in xylene for 5 min. Sections from human placentas were used as control slides.
Determination of PD-L1-positive staining
All sections were scored by two pathologists using a light microscope, and only stained tumor cell percentage was scored by the clinical standards for PD-L1 testing (14). Each pathologist was blinded to the staining protocols and scored the glass slides independently. Five high power fields of vision (400×) were randomly selected for each section, and the proportions of PD-L1-positive tumor cells was estimated as the number of stained tumor cells divided by the total number of tumor cells. Cases with <1% stained cells for SP142 and UMAB228 (1% cut-off), and with <25% stained cells for SP263 (25% cut-off) were considered negative (2,11).
Statistical analysis
The consistency of PD-L1 positivity using different PD-L1 antibodies was evaluated using kappa (κ) coefficients. According to the statistical criteria, κ within 0.4–0.75 is deemed as medium and high consistency, while κ <0.4 represents poor uniformity. Chi-square and Fisher’s exact tests were performed to evaluate the association between PD-L1 expression and clinic pathological factors. SPSS version 22 was employed for the statistical analysis. P<0.05 was considered statistically significant.
Results
PD-L1 expression
The PD-L1 IHC staining was detected at the membrane of tumor cells in all assays. The samples from 41 (48.8%), 51 (60.7%), and 50 (59.5%) patients were detected as PD-L1 positive in SP142, SP263, and UMAB228 assays, respectively. A comparison of PD-L1 staining performance was manifested by a heatmap on a case-by-case basis (Figure 1). Figure 2 demonstrates the NSCLC samples that represent a range of positive cellular rate for PD-L1 in all the three assays.
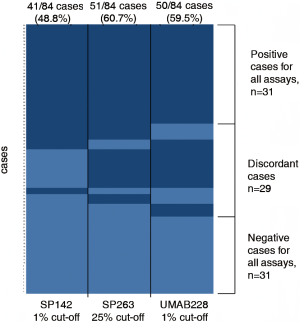
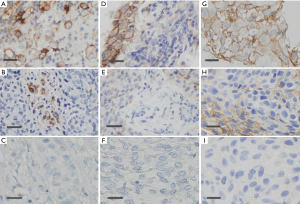
PD-L1 comparison using multiple PD-L1 antibodies
For antibody SP142 and UMAB228, 15 and 8 cases detected negative were stained positive by SP263, respectively. Meanwhile, 16 cases were tested negative by SP142 when deemed positive by UMAB228. Figure 3 reveals relevance among three PD-L1 IHC antibody assays by the PD-L1 status of 84 cases.
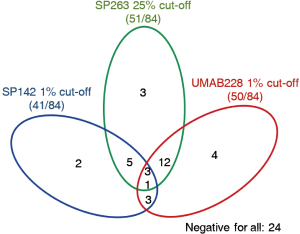
To quantify the potential concordance in the proportions of stained carcinoma cells, κ was calculated between every two antibodies. The value of κ was 0.53 [95% confidence interval (CI), 0.37–0.71], 0.46 (95% CI: 0.28–0.63), and 0.58 (95% CI, 0.39–0.76), respectively for SP142 vs. SP263, SP142 vs. UMAB228, and SP263 vs. UMAB228, demonstrating moderate concordance between every each antibody.
PD-L1 expression and clinicopathological characteristics
Table 2 represents the PD-L1 expression tested by three antibodies and the corresponding clinicopathological characteristics. Among the cases tested by SP142 and SP263, no significant correlation was observed between clinicopathological characteristics and PD-L1 expression. For cases detected by UMAB228, the univariate analysis revealed that PD-L1 expression was associated with gender (χ2=9.66, P=0.00) and smoking status (χ2=4.68, P=0.03).
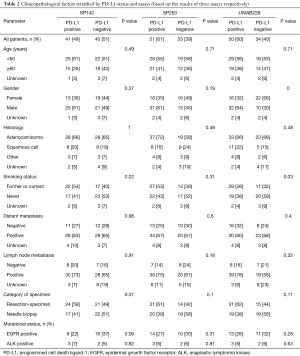
Full table
In addition, to exclude the false-negative and false-positive cases that might lead to inaccuracies in the study, we selected the consensus cases tested by three antibodies and re-analyzed using the same method. Among the concordant samples, 31 cases showed positive PD-L1 expression, while 24 cases showed negative. Table 3 exhibits the correlation between PD-L1 expression and smoking history (χ2=4.25, P=0.04), which is partly consistent to those tested by UMAB228.
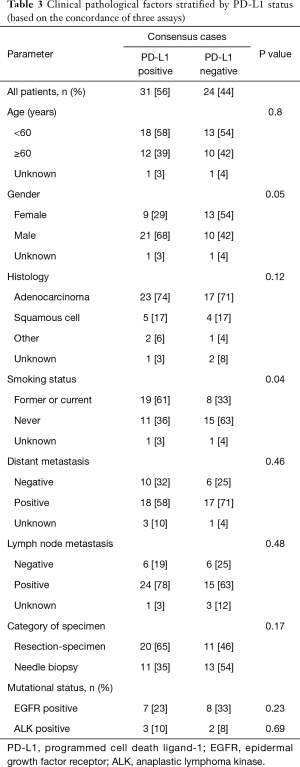
Full table
Discussion
Recent studies highlight the necessity of highly accurate, reliable IHC assays with great specificity and sensitivity. Among the three antibodies used in this study, SP142 is one of the most widely applied antibodies. The recent phase II POPLAR study and phase III OAK study showed that atezolizumab, with SP142 assay as a companion diagnostic, exhibited a survival benefit as compared to standard therapy in patients with NSCLC (1,15). The SP263 monoclonal antibody, raised against a synthetic peptide originated from the C-terminus of human PD-L1 protein, has also been used reproducibly in published clinical trials (16,17) and recently rendered as a complementary diagnostic for the assessment of PD-L1 expression. UMAB228 is a newly developed PD-L1 IHC antibody by Beijing OriGene Technologies, of which the specificity and sensitivity are demanded to be verified.
In the present study, moderate concordance was demonstrated between antibody SP263 and SP142, which have both been approved by FDA. However, SP263 showed a higher rate of tumor cell staining, suggesting the inherent difference among PD-L1 IHC antibodies. Ilie et al. (18) suggested that PD-L1 harbored only a small number of sites for binding the IHC antibodies as it contained only two hydrophilic regions, thereby making the IHC performance less efficient after slides fixed in formalin. Smith et al. (16) reported that several cases were assessed negative for PD-L1 expression with the E1L3N assay; however, with the SP263 assay, these cases displayed positive membrane staining. The Blueprint project conducted by Hirsch et al. (19) showed that the rate of positive staining by SP142 was lower than that with other antibodies including 28-8, 22C3, and SP263, which was partially consistent with our observations. Additionally, a German comparison study (20) came to the similar conclusion. Therefore, further studies are essential in order to determine whether SP263 is more preferable in clinical application.
UMAB228 is a newly developed PD-L1 IHC antibody, whose specificity and sensitivity remains to be verified. Our study indicated that UMAB228 exhibited concordance with both SP142 and SP263, suggesting the feasibility of UMAB228. However, due to the limited research available on UMAB228, more studies are required to explore its clinical value.
In addition, we demonstrated the relationship between PD-L1 expression and clinicopathological characteristics. The analysis of SP263 and SP142 cohort showed no significant correlation between PD-L1 expression and clinicopathological factors, which was consistent with the postulation of some other studies (12). However, PD-L1 expression tested by UMAB228 was significantly higher for males and patients with smoking history. Due to the inherent distinction of antibodies, the PD-L1 status evaluated by different assays may contribute to the varied conclusions.
To enable a reliable understanding of the potential association between PD-L1 status and clinicopathological characteristics, we selected consensus cases and analyzed by the same method. The results demonstrated correlation between PD-L1 expression and smoking status, which was partly consistent with the outcomes of UMAB228. Recent genetic analysis revealed that lung cancer from smokers exhibited 10-fold more somatic mutations than those from non-smokers (21). Another study suggested that for melanoma or non-small cell lung carcinoma, patients bearing higher level of somatic mutation showed elevated response rates to anti-PD-1/anti-PD-L1 inhibitors (22). Combining these findings with the current study, it can be speculated that smoking status might be valuable in identifying patients who might benefit from PD-1/PD-L1 inhibitors.
Nevertheless, the current study presents some limitations. One limitation is the lack of survival information and data for the response to PD-1/ PD-L1 inhibitors in this study. Secondly, the lack of a molecular gold standard for PD-L1 expression makes it difficult to unequivocally identify the positive cases and challenging to determine the prominence of the three antibodies. In further studies, we aspire to collect materials from more tumor types and a larger number of patients in order to provide recommendations for the optimization of PD-L1 testing.
In conclusion, these data may shed light on the convoluted status of PD-L1 biomarker testing. In the present study, the three antibodies demonstrated concordance with every each other, while SP263 exhibiting higher overall positive rate, based on the tumor cell staining results. The newly developed antibody UMAB228 showed concordance with both of the FDA-approved antibodies, SP142 and SP263. However, since relevant research is limited, more studies are essential for its optimization for clinical application. Besides, our study identified that PD-L1 positivity was significantly associated with smoking status. Moreover, the relationship between PD-L1 expression and clinicopathological characteristics might vary owing to the utilization of divergent antibodies, which suggests that the detection method requires intensive focus to achieve a specific conclusion and to guide the individualized treatment.
Acknowledgements
Funding: This study was funded by the National Natural Science Foundation of China (No. 81672982, 81101698) and Sichuan Provincial Research Foundation for Basic Research (No. 2016JY0050). We thank the Department of Pathology of West China Hospital for providing the assistance for this study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: According to the ethical requirement, the study was approved by West China Hospital of Sichuan University Biomedical Research Ethics Committee, with the number of the approval 20170426. Besides, participants involving in the study had given informed consent before taking part.
References
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- U.S. FDA. Durvalumab (Imfinzi). Available online: https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm555930.htm
- Hino R, Kabashima K, Kato Y, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 2010;116:1757-66. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Planchard D, Yokoi T, McCleod MJ, et al. A Phase III Study of Durvalumab (MEDI4736) With or Without Tremelimumab for Previously Treated Patients with Advanced NSCLC: Rationale and Protocol Design of the ARCTIC Study. Clin Lung Cancer 2016;17:232-6.e1. [Crossref] [PubMed]
- Cooper WA, Tran T, Vilain RE, et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer 2015;89:181-8. [Crossref] [PubMed]
- Mu CY, Huang JA, Chen Y, et al. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol 2011;28:682-8. [Crossref] [PubMed]
- Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori 2012;98:751-5. [PubMed]
- Zhong A, Xing Y, Pan X, et al. Prognostic value of programmed cell death-ligand 1 expression in patients with non-small-cell lung cancer: evidence from an updated meta-analysis. Onco Targets Ther 2015;8:3595-601. [Crossref] [PubMed]
- Takada K, Toyokawa G, Okamoto T, et al. A Comprehensive Analysis of Programmed Cell Death Ligand-1 Expression With the Clone SP142 Antibody in Non-Small-Cell Lung Cancer Patients. Clin Lung Cancer. 2017;18:572-82.e1. [Crossref] [PubMed]
- Sheffield BS, Fulton R, Kalloger SE, et al. Investigation of PD-L1 Biomarker Testing Methods for PD-1 Axis Inhibition in Non-squamous Non-small Cell Lung Cancer. J Histochem Cytochem 2016;64:587-600. [Crossref] [PubMed]
- McLaughlin J, Han G, Schalper KA, et al. Quantitative Assessment of the Heterogeneity of PD-L1 Expression in Non-Small-Cell Lung Cancer. JAMA Oncol 2016;2:46-54. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Smith J, Robida MD, Acosta K, et al. Quantitative and qualitative characterization of Two PD-L1 clones: SP263 and E1L3N. Diagn Pathol 2016;11:44. [Crossref] [PubMed]
- Igawa S, Sato Y, Ryuge S, et al. Impact of PD-L1 Expression in Patients with Surgically Resected Non-Small-Cell Lung Cancer. Oncology 2017;92:283-90. [Crossref] [PubMed]
- Ilie M, Hofman V, Dietel M, et al. Assessment of the PD-L1 status by immunohistochemistry: challenges and perspectives for therapeutic strategies in lung cancer patients. Virchows Arch 2016;468:511-25. [Crossref] [PubMed]
- Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol 2017;12:208-22. [Crossref] [PubMed]
- Scheel AH, Dietel M, Heukamp LC, et al. Harmonized PD-L1 immunohistochemistry for pulmonary squamous-cell and adenocarcinomas. Mod Pathol 2016;29:1165-72. [Crossref] [PubMed]
- Govindan R, Ding L, Griffith M, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell 2012;150:1121-34. [Crossref] [PubMed]
- Champiat S, Ferté C, Lebel-Binay S, et al. Exomics and immunogenics: Bridging mutational load and immune checkpoints efficacy. Oncoimmunology 2014;3:e27817-22. [Crossref] [PubMed]


