Application of 3D printing in the surgical planning of hypertrophic obstructive cardiomyopathy and physician-patient communication: a preliminary study
Introduction
Hypertrophic obstructive cardiomyopathy (HOCM) is a unique primary myocardial disease characterized by hypertrophy of the interventricular septum (IVS), a narrowed left ventricular outflow tract (LVOT) and, frequently, systolic anterior motion (SAM) of the mitral valve resulting in LVOT obstruction (1-3). Medical treatment is the first-line therapy for symptomatic patients with LVOT obstruction; however, left ventricular septal myectomy is the procedure of choice if medical treatment is unsuccessful or intolerable (4-6). Sometimes, the myectomy remains difficult due to the highly variable anatomy and complex physiology. A main challenge of myectomy is how to resect the IVS properly to achieve a satisfactory surgical goal: elimination or significant reduction of LVOT obstruction.
Three-dimensional (3D) printing is an emerging technology that enables creation of physical anatomic models from a patient’s imaging data. A 3D-printed prototype allows the surgeons direct visualization of a patient’s cardiac anatomy before surgery. This technique has been used in congenital heart disease for better understanding the complex anatomy and facilitating planning of operative methods (7,8). In the present study, 3D printing technology was applied to reconstruct the details of left heart in our 7 HOCM patients, surgical planning and the effectiveness of surgical treatment were evaluated.
Methods
General information
Seven consecutive HOCM patients received surgical treatment in our institute were enrolled in this study from Oct. 2015 to Mar. 2016. All patients were diagnosed by echocardiography. They were eligible for surgical procedure and met the following two criteria: (I) a LVOT gradient of ≥50 mmHg at rest or with physiological provocation; (II) unresponsive to maximum pharmacological therapy, including dyspnoea with New York Heart Association (NYHA) functional class II to IV and/or chest pain with Canadian Cardiovascular Society Angina class III or IV, and/or repetitive effort-related syncope despite prior appropriate medical therapy.
This study has been approved by the Institutional Review Board of Anzhen Hospital, which is affiliated with Capital Medical University (Beijing, China). A signed informed consent was obtained from each patient involved in this study.
3D Printing
Each patient was scanned by 64-slice spiral computed tomography (CT) scanner (GE Medical Systems, Chicago, IL, USA) in the supine position. The scan settings were as follows: a voltage of 120 kV, a current of 180–220 mA, and a slice thickness of 1 mm. The CT scan data of the heart were saved as DICOM files. DICOM formatted raw images were evaluated by the surgeon (Lai) to identify important anatomic features and regions of hypertrophy. Multilayer images were input into Mimics 19.0 (Materialize, Leuven, Belgium) to perform region reconstruction for 3D image segmentation. The computed imaging data were saved in STL (StereoLithography file) format using Mimics software and were output to a 3D printer (Objet350 Connex3, Stratasys Ltd., USA). Veroclear (Stratasys Ltd., USA), VeroCyan (Stratasys Ltd., USA), VeroMagenata (Stratasys Ltd., USA), Tango material (FlashForge Ltd., ZhengJiang, China) were used as the printing materials. Different colors can be achieved with a mixture of the two base colors-magenta and cyan. Thus, different anatomical structures can be represented with a special color for better identification. The printer consists of a plaster powder plunger and a building plunger. A roller transports a layer plaster powder from the plaster powder plunger to the building plunger. Then the print nozzles spray the adhesive on the segmented areas (regions of interest) in the building plunger and the first layer is completed. This process has to be repeated until the 3D-printed prototype is created. The plaster powder which were not segmented is removed with compressed air. After cleaning and drying of the prototype, a 1:1 ratio left heart prototype was obtained (Figure 1). Each prototype total process takes approximately 14–16 hours, almost all of which is unsupervised (preprocessing =1–2 h; printing =12–13 h; postprocessing <1 h).
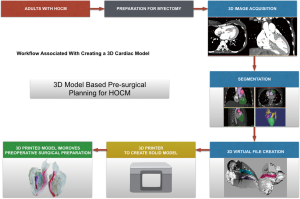
Assessment tool
Open questionnaire for patients and nonmedical professionals was designed to evaluate the role of 3D-printed models in the preoperative conversation. The questionnaire included four questions: (I) Is the 3D-printed prototype useful for helping you to understand the surgical plan? (II) Is the 3D-printed prototype useful to obtain a clear understanding of your condition? (III) Would you like the doctor to use a 3D-printed prototype to communicate with you? (IV) How do you access your overall satisfaction of the preoperative conversation? The scores ranged from 1 to 10 points; one point indicated that the model was useless, and ten points indicated the model was very useful.
The surgeon used the 3D-printed left heart prototype to inform the patient and their relatives about the patient’s conditions so that they could understand their diseases, the surgical treatment strategy, and the potential surgical result. After the conversation, the patients and their relatives finished the questionnaire to evaluate the effectiveness of the conversation, and indicate their preference for communicating with the surgeon with a 3D-printed heart prototype.
Surgical techniques
Septal myectomy was performed through a transverse aortotomy extended into the noncoronary sinus. The continuous resection was commenced at the nadir of the right aortic sinus leftward toward the mitral valve annulus and apically to the base of the papillary muscles. All areas of papillary muscle fusion to the septum or ventricular free wall were divided, and anomalous chordal structures and fibrous attachments of the mitral leaflets to the ventricular septum or free wall were divided or excised.
LVOT gradient measurement
Intraoperative transesophageal echocardiography (TEE) was routinely performed. Transgastric views were used to measure LVOT velocity by continuous-wave Doppler before and after operation. Peak gradients were calculated from optimal spectral Doppler envelopes and velocities calculated according to the formula 4V2.
Follow-up
Patients were followed-up at 3 months after operations. The recommended measures included physical examination and TEE. Postoperative complications and the early outcomes were recorded. The follow-up study was carried out by subsequent clinic visits to the outpatient departments.
Results
The baseline clinical variables of HOCM patients are shown in Table 1. Five patients reported NYHA class III/IV heart failure symptoms. Syncope history was present in four patients. The mean septal thickness and LVOT gradient derived from TEE were 21.57±4.65 mm and 82.71±31.63 mmHg, respectively. The SAM and mitral valve insufficiency were present in all patients and the average mitral valve regurgitation area was 6.00±2.51 cm2 (Table 1).

Full table
3D print prototypes were used for left heart reshaping pre- and post-operation for all patients (Figures 2,3). The left ventricular and the aorta were divided into two parts along left heart long axis for better vision of hypertrophic IVS, and narrowed LVOT. The papillary muscles were colored red and green. The SAM of mitral valve was obvious in the model which significantly aggravated obstruction of LVOT.
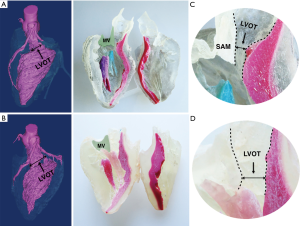
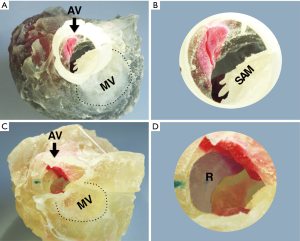
Septal myectomy was performed in all patients. The mean weight of resected muscle sample was 11.14±2.85 g and the mean cross-clamp time is 51.85±9.65 min. Septal thickness and peak gradient were 17.42±5.88 mm and 14.91±6.89 mmHg, respectively. SAM disappeared in all patients, and mitral valve regurgitation was no or trivial in five cases and mild in two patients postoperatively (Table 1).
Questionnaire demonstrated patients and their relatives exhibited a high degree of satisfaction with the use of a 3D-printed prototype for doctors to explain the details of this disease (Table 2).

Full table
Discussion
Many imaging techniques, such as echocardiography, CT and magnetic resonance imaging (MRI), can create 2D images of the heart. An obvious progress that allows better visualisation and understanding of the spatial anatomical relations would be the transition from 2D to 3D. 3D echocardiography is currently becoming prevalent in clinical practice (9). CT and MRI techniques used for 3D reconstruction (10) are emerging as valuable tools in the interventional cardiology (11). These imaging advances allow clinical evaluation more accurate, however, they are just a virtual 3D. 3D print is a new technology that could represent complex 3D relationships veritably. 3D printing technology is making significant progress in helping surgeons to think about surgical manners preoperatively, and anticipate technical challenges that may be encountered (12).
3D printing technology had been used and reported in congenital heart disease and great vessel disease. Farooqi et al. (13) described the use of 3D printing to guide ventricular assist device placement in adults with congenital heart disease and heart failure. Anwar et al. (14), introduce four examples of complex congenital heart diseases with 3D technology. Jacobs et al. (15), reported 3D models were also used to design the resection of a ventricular aneurysm and heart tumor. Yang et al. (16) reported one case of 3D-printed hypertrophic cardiomyopathy heart model used in myectomy from an apical incision.
3D-printed models were constructed preoperatively and postoperatively in seven HOCM patients undergoing septal myectomies through aortotomy in this study. The essential details affecting the outcomes of the myectomies are well demonstrated and better understanding preoperatively. In this scheme, the 3D-print models were constructed on a 1:1 scale, which allowed surgeons practice operative rehearsal from aorta view in the models. Furthermore, the 3D print model could be turned over from all views, which was very helpful for surgeon to design individual surgical proposal, according to different types of hypertrophic IVS and subvalvular abnormalities. SAM of mitral valve often contributes to LVOT obstruction in HOCM patients. About 66% of HOCM patients have structural abnormalities of mitral valve, including enlarged leaflet area, leaflet elongation, or anomalous papillary muscle insertion into the anterior mitral leaflets. These anomalies that can lead to residual pressure gradient after the myectomy if not recognized and managed (17). 3D-print model could demonstrate these abnormalities intuitively, which could instruct the surgeon to correct the associated anomalies. Young surgeon with less experience of myectomy might make mistakes of inadequate resection, and residual pressure gradient might present after operation. The postoperative 3D heart model could verify the resection was sufficient or not, in comparison of the preoperative model. This process is helpful to check the surgical result and improve their techniques for better outcomes.
3D-printed prototype of the heart for illustrating the heart anomalies and surgical strategy received satisfactory outcomes. furthermore, it was also very helpful for patients and family members to understand their conditions, and provide a useful tool for an effective communication.
Limitations
This preliminary study is limited to only seven HOCM patients. There are no comparison groups using other heart models in this study. Sometimes, the technicians of 3D print can not understand the surgeons' thought accurately, and some valuable structures can not be demonstrated clearly in the model. The relationship of 3D print anatomy and hemodynamic results recorded by echocardiography will be investigated in our further study.
In conclusion. Our preliminary study illustrates 3D-printed models derived from CT scans are feasible in patients with HOCM. It is a useful tool in individualized planning for myectomies and represent an effectively tool for physician-patient communication.
Acknowledgements
Funding: This study was supported by a grant from the National Natural Science Foundation of China (81770371, 81370328).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study has been approved by the Institutional Review Board of Anzhen Hospital, which is affiliated with Capital Medical University (Beijing, China). A signed informed consent was obtained from each patient involved in this study.
References
- Wigle ED. Novel insights into the clinical manifestations and treatment of hypertrophic cardiomyopathy. Curr Opin Cardiol 1995;10:299-305. [Crossref] [PubMed]
- Wigle ED, Rakowski H, Kimball BP, et al. Hypertrophic cardiomyopathy. Clinical spectrum and treatment. Circulation 1995;92:1680-92. [Crossref] [PubMed]
- Wigle ED, Sasson Z, Henderson MA, et al. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog Cardiovasc Dis 1985;28:1-83. [Crossref] [PubMed]
- Cohn LH, Trehan H, Collins JJ Jr. Long-term follow-up of patients undergoing myotomy/myectomy for obstructive hypertrophic cardiomyopathy. Am J Cardiol 1992;70:657-60. [Crossref] [PubMed]
- Schulte HD, Borisov K, Gams E, et al. Management of symptomatic hypertrophic obstructive cardiomyopathy--long-term results after surgical therapy. Thorac Cardiovasc Surg 1999;47:213-8. [Crossref] [PubMed]
- Vriesendorp PA, Schinkel AF, Soliman OI, et al. Long-term benefit of myectomy and anterior mitral leaflet extension in obstructive hypertrophic cardiomyopathy. Am J Cardiol 2015;115:670-5. [Crossref] [PubMed]
- Shiraishi I, Yamagishi M, Hamaoka K, et al. Simulative operation on congenital heart disease using rubber-like urethane stereolithographic biomodels based on 3D datasets of multislice computed tomography. Eur J Cardiothorac Surg 2010;37:302-6. [PubMed]
- Kiraly L, Tofeig M, Jha NK, et al. Three-dimensional printed prototypes refine the anatomy of post-modified Norwood-1 complex aortic arch obstruction and allow presurgical simulation of the repair. Interact Cardiovasc Thorac Surg 2016;22:238-40. [Crossref] [PubMed]
- Lang RM, Badano LP, Tsang W, et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging 2012;13:1-46. [Crossref] [PubMed]
- Kaji S, Nasu M, Yamamuro A, et al. Annular geometry in patients with chronic ischemic mitral regurgitation: three-dimensional magnetic resonance imaging study. Circulation 2005;112:I409-14. [PubMed]
- Schwartz JG, Neubauer AM, Fagan TE, et al. Potential role of three-dimensional rotational angiography and C-arm CT for valvular repair and implantation. Int J Cardiovasc Imaging 2011;27:1205-22. [Crossref] [PubMed]
- Johnston NF, Prendiville T, McMahon CJ. 3D printing of severe hypertrophic cardiomyopathy in a child with Rasopathy. Ir J Med Sci 2018;187:55-7. [Crossref] [PubMed]
- Farooqi KM, Saeed O, Zaidi A, et al. 3D printing to guide ventricular assist device placement in adults with congenital heart disease and heart failure. JACC Heart Fail 2016;4:301-11. [Crossref] [PubMed]
- Anwar S, Singh GK, Varughese J, et al. 3D printing in complex congenital heart disease: across a spectrum of age, pathology, and imaging techniques. JACC Cardiovasc Imaging 2017;10:953-6. [Crossref] [PubMed]
- Jacobs S, Grunert R, Mohr FW, et al. 3D-Imaging of cardiac structures using 3D heart models for planning in heart surgery: a preliminary study. Interact Cardiovasc Thorac Surg 2008;7:6-9. [Crossref] [PubMed]
- Yang DH, Kang JW, Kim N, et al. Myocardial 3-Dimensional Printing for Septal Myectomy Guidance in a Patient With Obstructive Hypertrophic Cardiomyopathy. Circulation 2015;132:300-1. [Crossref] [PubMed]
- Klues HG, Maron BJ, Dollar AL, et al. Diversity of structural mitral valve alterations in hypertrophic cardiomyopathy. Circulation 1992;85:1651-60. [Crossref] [PubMed]


