Erythromycin poudrage versus erythromycin slurry in the treatment of refractory spontaneous pneumothorax
Introduction
Spontaneous pneumothorax (SP) is a significant problem with an incidence of 24.00 per 100,000 each year for men and 9.80 per 100,000 each year for women in England (1). A high recurrence rate was found within two years after the first attack of pneumothorax (2,3). The ipsilateral recurrence rate of SP after a first attack ranges within 20–60% without preventative measures (4-6), and subsequent recurrence rates gradually increase, up to 62% for a second recurrence and 83% for a third recurrence (4,5). Three important factors are associated with the recurrence of pneumothorax: high height/weight ratio, the presence of underlying lung diseases, and non-use of a pleurodesis agent (7). Refractory spontaneous pneumothorax (RSP) includes recurrent SP (unilateral pneumothorax occurrence ≥2 times or bilateral pneumothorax occurrence ≥3 times) and persistent SP (persistent air leakage after chest tube drainage >7 days (8) and lack of reexpansion on chest X-ray), and both require further corrective and/or preventive measures other than pleural drainage (9,10) .
The therapeutic options of RSP include continuous chest drainage and interventional approaches, such as thoracotomy with pleurectomy and bullae resection or video-assisted thoracoscopic surgery (VATS) with bullae resection plus pleural abrasion. Pleurodesis using sclerosing agents has become a significant therapy in the treatment of RSP on account of its high efficiency and safety (11,12). Moreover, several studies have reported that intrapleural administration of a sclerosing agent after drainage via a chest tube or a thoracoscope is an effective method that significantly reduces the recurrence incidence of SP (13,14).
Erythromycin was first found to be as effective as tetracycline (TCN) for pleurodesis in rabbits, according to a study conducted by Carvalho (15). Since then, several researches (16,17) in animal models have demonstrated that erythromycin was an ideal sclerosing agent, accompanied with diffuse fibrosis without significant inflammatory reactions. A series of clinical studies have been published, advocating erythromycin instillation via a chest tube (‘erythromycin slurry’, ES) as a simple and effective technique in the treatment SP (18-20). Thoracoscopically insufflated erythromycin (‘erythromycin poudrage’, EP) was carried out by Hong B (21) and discovered that thoracoscopic erythromycin poudrage to be an efficient method in SP. Nevertheless, the appropriate mode of erythromycin administration as a sclerosing agent remains unknown and no related report could be found.
We conducted this study to compare erythromycin poudrage under thoracoscopy with ES through a chest tube, investigating its efficacy and safety in the treatment of RSP.
Methods
Patients
A total of 57 patients diagnosed with RSP in the Department of Respiratory Medicine of Shandong Provincial Hospital affiliated to Shandong University from 2004 to 2016 for chest drainage and pleural adhesion were included into the study. Among these patients, 41 patients had recurrent SP and 16 patients had persistent SP. All patients refused a surgical operation as the first line treatment. Exclusion criteria: pregnancy patients, and patients with coagulation disorders, history of erythromycin allergy, cardiac arrhythmia, renal insufficiency, hepatic dysfunction and electrolyte imbalance. The following data were available from each patient: general examination, laboratory investigations and radiological evaluation with chest X-ray radiograph (CXR) or chest computed tomography (CT) scan.
The Ethics Committee of Shandong Provincial Hospital affiliated to Shandong University approved the study protocol which was conducted according to the principle expressed in the Declaration of Helsinki. All patients have provided written informed consents.
The following clinical variables were recorded: data on demographics, types of SP, procedural results, procedure-related complications, duration of post-procedural chest tube drainage, post-procedural hospitalization time, and outcome of follow-up.
Techniques
Erythromycin poudrage was performed under medical thoracoscopy (MT) in the endoscopy suite with aseptic environment. All patients accepted oxygen by nasal catheter at a rate of 4 L/min with continuous electrocardiograph and percutaneous oximetry monitoring. Patients were placed in the lateral decubitus position with conscious sedation using diazepam or benzodiazepine. The lateral area of chest was sterilized and draped, and 10 mL of 1% lidocaine (Silver Lake Shiyao Pharmaceutical Co.Ltd; Yuncheng, Shanxi Province, China) with 0.5 mg of epinephrine [GrandPharma (China) Co., Ltd.; Wuhan, Hubei Province] was administered to the selected intercostal space for local anesthesia. An 11-mm trocar (Olympus; Germany; WA58365S) was inserted in the intercostal space, the rigid thoracoscope (Olympus; Germany) was attached to a video camera, and monitor (Sony Corporation; China) was inducted through the trocar to inspect the whole pleural cavity (Figure 1). A second incision was chosen depending on the intrathoracic condition, and the second trocar (5.5 mm diameter, Olympus; Germany; A5948) was introduced under the vision of thoracoscope. Then, 1 g of sterile erythromycin (Hunan Kelun Pharmaceutical Co., Ltd; Hunan, China) was insufflated into the pleural cavity with a catheter and bulb syringe, which was uniformly distributed (Figure 2). A 28F chest tube was inserted and connected to a 20 cm/H2O suction until no air overflowed. At that time, the chest tube was clamped for 24 hours and a chest X-ray was performed to evaluate the re-expansion of the lung. The chest tube was removed, and the time of chest tube removal was recorded. The criterion of immediate success was determined by chest radiographs, as follows: complete re-expansion of the lung and no residual gas in the pleural space.


The ES was performed at the bedside. Then, 5–10 mL of 1% lidocaine was injected into the pleural cavity through a 20 F chest tube, followed by the infusion of 1 g of erythromycin in 40 mL of 5% glucose solution into the pleural cavity. The chest tube was clamped for 24 hours, and the patients turned around in different positions. When patients suffered from dyspnea or discomfort, the drain was kept open after the procedure. After 24 hours, the chest tube was connected to a 20 cmH2O suction, and was monitored daily for cessation of the air leak. At that time, the chest tube was clamped for 24 hours and a chest X-ray was obtained to ensure the expansion of the lung prior to removal. The timing of chest tube removal was recorded.
Follow-ups lasted for 3 years or were completed until December 2016. These patients were regularly seen at ambulatory visits. Chest roentgenograms were obtained one month after the procedure and monthly for three months. Further follow-up was achieved every 3 months for 1 year and every 6 months for 2 years. Recurrent pneumothorax was identified by case notes and imaging findings. A recurrent pneumothorax is defined as a pneumothorax on CXR greater than 10% of the ipsilateral hemithorax, and the time of recurrence was recorded.
Statistical analysis
The descriptive analysis was expressed as frequency and mean ± SD. Continuous variables were subject to normal distribution and compared with t-test. Chi-square test was performed to compare categorical variables and Fisher’s exact test was used for small samples. A P<0.05 was considered statistically significant. Data were analyzed using SPSS 19.0 statistical software (SPSS Inc., Chicago, IL, USA).
Results
The present study included 57 patients with 30 undergoing EP and 27 undergoing ES. Patient demographic data were present in Table 1. Fifty-two males and five females with a mean age of 51.84 years old (±14.67 years) were included in the study. Recurrent SP was found in 41 patients (71.93%) with a frequency of more than two times per person, while one patient experienced it 12 times. The duration of recent SP was assessed from the presentation of recent pneumothorax until pleurodesis, which was based on the chest radiogram. Thirty-five patients underwent chest drainage for 3–58 days before hospitalization, with a result of persistent air leakage. The classification of SPs was divided into <20%, 20–50% and >50%, which was based on the average interpleural distance acquired from frontal and lateral CXR (24).

Full table
Table 2 summarized the demographic and clinical characteristics of these two groups, and no difference was found in clinical features between the EP and ES groups.

Full table
There was no difference between the two treatment methods with erythromycin in the rate of immediately successful pleurodesis (P=0.087, Table 3). In EP group, postprocedural air bubbling stopped within five days in 24 patients (80%). Six EP patients had air leak for at least six days and they all underwent recurrent SP. These patients refused surgical treatment, and were managed with continuous chest drainage. In 16 of 27 ES patients (59.26%), postprocedural bubbling stopped within five days. In 11 ES patients, postprocedural air leakage lasted for six days or more, but ceased with continuous thoracic drainage. The chest tube was removed after 6.23±3.04 days in EP patients, and after 10.67±9.81 days in ES patients (P=0.032, Table 3). No procedure-related mortality was found in both techniques. The effectiveness in both groups was not influenced by gender, age, smoking, underlying lung diseases, pneumothorax episodes, radiological findings and the classification of SPs in CXR.

Full table
Table 4 summarized the common complications after both procedures and no significant statistical difference was found between these two groups. The most common postprocedural complication was chest pain, which occurred in 23 EP patients (76.67%) and 17 ES patients (62.96%) (P=0.259). In the EP group, 17 patients were given analgesic drugs and chest pain was relieved within two days. In the ES group, all patients with chest pain required analgesic drugs and a significant statistical difference was found between the two groups (P=0.03). Twenty-two of 30 EP patients (73.33%) had fever with a temperature >37.5 °C while 18 patients (66.67%) in the ES group had a temperature >37.5 °C (P=0.583). Six patients in the EP and four patients in the ES group had a temperature >38.5 °C and temperature dropped to normal level with hormone drugs or non-steroidal anti-inflammatory drugs (NSAIDS). Pleurodesis-related dyspnea was found in 5 of 30 EP patients (16.67%) and in eight of 27 ES patients (29.63%) (P=0.244). Dyspnea was alleviated in all patients with oxygen by nasal catheter at a rate of 3–5 L/min. Prolonged air leak (>2 days) occurred in 15 patients (50%) in the EP group and 18 patients (66.67%) in the ES group (P=0.203). The air leak ceased with effective treatments before these patients were discharged from the hospital. Three EP patients (10%) and four ES patients (14.81%) had subcutaneous emphysema, which did not require surgical intervention (P=0.882). Two patients (6.67%) in the EP group and five patients (18.52%) in the ES group developed cough and relieved without medication (P=0.339). No procedure-related mortality was found in both treatments.

Full table
The outcome of the follow-ups and the comparison between the ES and EP groups were present in Table 5. There was no significant statistical difference between these two techniques at any time from the pleurodesis. After 1 month, no recurrent pneumothorax was found in both groups. After two months, no recurrent pneumothorax was observed in the EP group, while recurrent pneumothorax was observed in one patient in the ES group (P=0.4). After 12 months, two patients had recurrent pneumothorax in the EP group and one patient experienced recurrent pneumothorax in the ES group (P=1.0). Twenty-four months after the procedure, three patients in the EP group and three patients in the ES group developed recurrent pneumothorax (P=0.928). At the end of follow-up, recurrent pneumothorax was found in 5 of 24 EP patients and in 5 of 16 ES patients (P=0.709). There was no mortality during the follow-up.
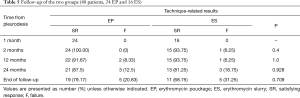
Full table
Discussion
The treatment of RSP has been a significant problem for physicians and surgeons. The aim of therapy in patients with RSP is to achieve reexpansion of lung and prevent the recurrence of pneumothorax. With rapidly evolving technology, the safety and efficacy of each therapeutic intervention have become essential conditions for patients with RSP. Pleurodesis has been demonstrated to be an effective and safe therapy in solving these mentioned problems. Several reports have certified that the recurrence rates of SP can be reduced with the intrapleural application of sclerosing agents (13,14). In the present study, 57 patients with RSP underwent pleurodesis with erythromycin. The success rates were 80% in the EP group and 59.26% in the ES group, and the recurrence rates were 12.5% in the EP group and 18.75% in the ES group after two years, which was similar to previous trials and case series (25,26).
Zeng et al. first evaluated the value of erythromycin as a pleural sclerosant in patients with SP (18). In that study, 1 g of erythromycin in 50 mL of 0.90% saline was injected into the pleural cavity of 47 patients with SP. After treatment, the total response rate was 85.10%; and after the first injection, the efficacy rate was 57.40%. The recurrence rate after 12–24 months was 10.30%, and the most common side effect was chest pain (42.50%). Since then, several studies have found that the success rates of erythromycin pleurodesis for slurry in RSP ranged from 52.70% to 53.10% at the first time, and the total success rates ranged from 81.30% to 84.40%, with recurrence rates ranging 10.40–11.10% after 1 year (19,20). In the present study, the efficacy of ES in RSP for the first time was 59.26%, and the recurrence rate after 1-year follow-up was 6.25%, which was better than previous articles.
Pleurodesis under MT has been widely applied because it allows the sclerosant to be thoroughly distributed on the whole pleural surface. A study conducted by Hong et al, was performed in 31 RSP patients who were treated with EP by thoracoscopy .The results revealed an efficiency of 76.67% without significant adverse effects, and the recurrence rate after 24 months was 13.33% (21). Furthermore, we found that the success rate in the EP group was 80% and the recurrence rate after 24 months was 12.5%, which was similar with previous studies. To our knowledge, the determination of which mode of erythromycin application has more advantages in the treatment of RSP has not been reported in any study.
The present study revealed that there was no difference in immediate success rate between the EP and ES groups. However, patients in the EP group had a shorter duration of postprocedural chest tube drainage (P=0.032). We considered that EP is more efficient than ES. It can be explained that EP allows erythromycin to be thoroughly distributed on the whole pleural cavity and results in stronger adherence reactions. Moreover, the erythromycin solution in ES may be drained from the pleural cavity and delay the timing of chest tube removal.
During the follow-up, no mortality occurred, and the difference in recurrences between the EP and ES groups was not statistically significant. In the EP group, recurrent pneumothorax occurred in five patients and the recurrence time was 4, 5, 18, 26 and 34 months after the procedure. All patients were accompanied with underlying lung diseases and were diagnosed with pneumothorax by chest radiograph. Five ES patients developed pneumothorax after follow-up and 3 patients had a history of underlying lung diseases. This suggests that the recurrence rate is probably higher in secondary spontaneous pneumothorax which has been described in several published studies (27,28).
The exact mechanism of pleurodesis by erythromycin remains not completely understood. Xie et al., took a trial to explore the mechanism by which erythromycin produced pleurodesis (29). They found that erythromycin injures human pleural mesothelial cells (HPMCs) in a dose- and time-dependent manner; and as a result, it promoted the secretion of TNF-α and TGF-β1. Moreover, erythromycin decreased the level of connexin43 in HPMCs, which may affect the response of HPMCs to pleurodesis with erythromycin.
Severe adverse effects after the use of intravenous and oral erythromycin are rare. Some studies have even reported the occurrence of ventricular tachycardia (30) and allergic reactions. Reversible hearing loss ever generally occurs in patients with renal insufficiency and in patients receiving high doses of erythromycin in isolated reports. However, none of the side effects mentioned above occurred in the present study. Post-procedural complications predominantly included fever, chest pain and prolonged air leak which were also discovered in previous articles (31,32). There was no difference in the incidence of these complications between these two groups. However, chest pain in the ES group is more severe. The reason may be that erythromycin is not evenly distributed in the pleural cavity, and high concentrations of erythromycin could stimulate the parietal pleura and induce severe chest pain. Chest pain either from the sclerosing agent or the chest tube can both be relieved through the application of systemic narcotics. We found that when we added local anesthetics to the slurry (<10 mL of 1% lidocaine), post-pleurodesis pain could be obviously lessened. In most patients, chest pain was slight, short lasting and easy to control. Although fever (T>38.5 °C) was observed within 48 hours in six patients (20%) in the EP group and four patients (14.81%) in the ES group, post-procedural empyema was not found, and fever was controlled by the administration of physical cooling and NSAIDs. Chest congestion was mild with SpO2 >90%, which had no influence on the procedures. This was quickly relieved with oxygen through the nasal catheter. The incidence of subcutaneous emphysema observed in the present study was not high, and this minor complication, which attributed to coughing during parietal pleural manipulation, was resolved without intervention. It was considered that the administration of 1% lidocaine into the pleural space ahead of the trocar insertion can avoid this adverse reaction (33).
Although EP is more effective than ES, the appropriate mode of erythromycin administration for a RSP patient depends on several considerations. Compared with ES, EP has been considered by some patients to have a higher cost, longer treatment time and greater medical security, which may affect the treatment preferences of patients who pay more attention to their quality of life. Furthermore, ES at the bedside is a simpler and less invasive procedure with possibly lower cost. We recognized that our sample size was small. Hence, a larger, prospective, randomized controlled research is needed to verify our results. Moreover, the mechanism of erythromycin in the treatment of pneumothorax requires further research.
In conclusion, erythromycin is an efficient sclerosing agent for pleurodesis in patients with RSP. Both ES and EP are effective and safe technologies. Furthermore, EP is more efficient than ES.
Acknowledgements
We thank the staff at the Department of Respiratory Medicine of Shandong Provincial Hospital affiliated to Shandong University for their assistance in performing clinical operations and collecting clinical data. There was non-financial support in our article.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Ethics Committee of Shandong Provincial Hospital affiliated to Shandong University approved the study protocol which was conducted according to the principle expressed in the Declaration of Helsinki. All patients have provided written informed consents.
References
- Gupta D, Hansell A, Nichols T, et al. Epidemiology of pneumothorax in England. Thorax 2000;55:666-71. [Crossref] [PubMed]
- Sadikot RT, Greene T, Meadows K, et al. Recurrence of primary spontaneous pneumothorax. Thorax 1997;52:805-9. [Crossref] [PubMed]
- Chiu CY, Chen TP, Wang CJ, et al. Factors associated with proceeding to surgical intervention and recurrence of primary spontaneous pneumothorax in adolescent patients. Eur J Pediatr 2014;173:1483-90. [Crossref] [PubMed]
- Elfeldt RJ, Schroder DW, Thies J. Long-term follow-up of different therapy procedures in spontaneous pneumothorax. J Cardiovasc Surg (Torino) 1994;35:229-33. [PubMed]
- Krasnik M, Stimpel H, Halkier E. Treatment of primary spontaneous pneumothorax with intrapleural tetracycline instillation or thoracotomy. Follow-up of management program. Scand J Thorac Cardiovasc Surg 1993;27:49-51. [Crossref] [PubMed]
- Vernejoux JM, Raherison C, Combe P, et al. Spontaneous pneumothorax: pragmatic management and long-term outcome. Respir Med 2001;95:857-62. [Crossref] [PubMed]
- Guo Y, Xie C, Rodriguez RM, et al. Factors related to recurrence of spontaneous pneumothorax. Respirology 2005;10:378-84. [Crossref] [PubMed]
- Tschopp JM, Brutsche M, Frey JG. Treatment of complicated spontaneous pneumothorax by simple talc pleurodesis under thoracoscopy and local anaesthesia. Thorax 1997;52:329-32. [Crossref] [PubMed]
- Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest 2001;119:590-602. [Crossref] [PubMed]
- Henry M, Arnold T, Harvey J. BTS guidelines for the management of spontaneous pneumothorax. Thorax 2003;58 Suppl 2:ii39-52. [Crossref] [PubMed]
- Cardillo G, Carleo F, Giunti R, et al. Videothoracoscopic talc poudrage in primary spontaneous pneumothorax: a single-institution experience in 861 cases. J Thorac Cardiovasc Surg 2006;131:322-8. [Crossref] [PubMed]
- Cardillo G, Facciolo F, Giunti R, et al. Videothoracoscopic treatment of primary spontaneous pneumothorax: a 6-year experience. Ann Thorac Surg 2000;69:357-61; discussion 361-2. [Crossref] [PubMed]
- Chen JS, Chan WK, Tsai KT, et al. Simple aspiration and drainage and intrapleural minocycline pleurodesis versus simple aspiration and drainage for the initial treatment of primary spontaneous pneumothorax: an open-label, parallel-group, prospective, randomised, controlled trial. Lancet 2013;381:1277-82. [Crossref] [PubMed]
- Olavarrieta JR, Coronel P. Expectations and patient satisfaction related to the use of thoracotomy and video-assisted thoracoscopic surgery for treating recurrence of spontaneous primary pneumothorax. J Bras Pneumol 2009;35:122-8. [Crossref] [PubMed]
- Carvalho P, Knight LL, Olson RD, et al. Effects of erythromycin on the rabbit pleura: its potential role as a pleural sclerosant. Am J Respir Crit Care Med 1995;151:1228-32. [PubMed]
- Hashemzadeh S, Hashemzadeh K, Mamaghani K, et al. Pleurodesis by erythromycin, tetracycline, Aerosil™ 200, and erythromycin plus Aerosil™ 200 in a rat model: a preliminary study. Daru 2012;20:79. [Crossref] [PubMed]
- Miller Q, Meschter C, Neumaster T, et al. Comparison of pleurodesis by erythromycin, talc, doxycycline, and diazepam in a rabbit model. J Surg Educ 2007;64:41-5. [Crossref] [PubMed]
- Zeng M, Xie CM, Wu JF. The value of erythromycin pleurodesis in the treatment of spontaneous pneumothorax. Chin J Respir Crit Care Med 2004;3:110-2.
- Fang WJ. The value discussion of erythromycin pleurodesis in the treatment of refractory pneumothorax. Pract Clin Med 2009;10:51-2.
- Wei J, Liu QZ. The study of erythromycin pleurodesis in the treatment of refractory pneumothorax. Pract Clin Med 2009;10:30.
- Hong B, Huang LH. Clinical observation of erythromycin power pleurodesis under thoracoscope in the treatment of refractory spontaneous pneumothorax. Chongqing Med J 2015;44:1964-6.
- Zhai CC, Lin XS, Yao ZH, et al. Pneumothorax. Asvide 2018;5. Available online: http://asvidett.amegroups.com
- Zhai CC, Lin XS, Yao ZH, et al. Erythromycin poudrage. Asvide 2018;5. Available online: http://asvidett.amegroups.com
- Rhea JT, DeLuca SA, Greene RE. Determining the size of pneumothorax in the upright patient. Radiology 1982;144:733-6. [Crossref] [PubMed]
- Li C. Erythromycin-induced pleural adhesions in the treatment of refractory spontaneous pneumothorax. J Trop Med 2008;8:828-9.
- Zhou CY. The value of erythromycin pleurodesis in the treatment of obstinate desease of pleura. J Clin Pulm Med 2007;12:595-6.
- Light RW, O'Hara VS, Moritz TE, et al. Intrapleural tetracycline for the prevention of recurrent spontaneous pneumothorax. Results of a Department of Veterans Affairs cooperative study. JAMA 1990;264:2224-30. [Crossref] [PubMed]
- Archer GJ, Hamilton AA, Upadhyay R, et al. Results of simple aspiration of pneumothoraces. Br J Dis Chest 1985;79:177-82. [Crossref] [PubMed]
- Xie C, Huang JQ, Light RW. The effects of erythromycin on the viability and the secretion of TNF-alpha and TGF-beta1 and expression of connexin43 by human pleural mesothelial cells. Respirology 2005;10:567-71. [Crossref] [PubMed]
- Gitler B, Berger LS, Buffa SD. Torsades de pointes induced by erythromycin. Chest 1994;105:368-72. [Crossref] [PubMed]
- Balassoulis G, Sichletidis L, Spyratos D, et al. Efficacy and safety of erythromycin as sclerosing agent in patients with recurrent malignant pleural effusion. Am J Clin Oncol 2008;31:384-9. [Crossref] [PubMed]
- Liu XH, Pan XJ, Yang ZH. Effect of erythromycin on persistent air-leak Spontaneous Pneumothorax. J Clin Pulm Med 2008;13:187-8.
- Nezu K, Kushibe K, Tojo T, et al. Thoracoscopic wedge resection of blebs under local anesthesia with sedation for treatment of a spontaneous pneumothorax. Chest 1997;111:230-5. [Crossref] [PubMed]


