Establishment of animal model of gastroesophageal reflux disease by per-oral endoscopic tunneling: a preliminary study
Introduction
Gastroesophageal reflux disease (GERD) refers to the symptoms and complications induced by the reflux of contents of the stomach into the esophagus, oral cavity (including the throat), or lung. GERD is a common disorder of upper gastrointestinal motility, which seriously threatens human health, especially in Western developed countries (1). The mechanism of this disease is the dysfunction of the lower esophageal sphincter (LES) induced by the decrease of the anti-reflux mechanism, the enhancement of damage of reflux content to the esophageal mucosa, and the decrease in esophageal acid clearance. In addition, the high pressure region of the gastroesophageal junction is considered to be the key factor to prevent reflux. Continuous reflux of the stomach and duodenal fluid has great influence on the daily life of an individual. Studies have revealed that approximately 10% of these patients develop reflux esophagitis, Barrett’s esophagus and lower esophageal adenocarcinoma (2-5). Proton-pump inhibitor therapy is the main treatment method for GERD, which can relieve symptoms in most patients, and show a very good curative effect. However, this treatment only controls its symptoms instead of curing it, and its curative effect on refractory GERD is poor (6). Anti-reflux surgery is one of the best options for severe GERD patients undergoing long-term treatment. At present, endoscopic treatment cannot replace traditional medicine or surgical treatment due to efficacy and safety problems. Therefore, a simple, safe and effective GERD therapy is needed. We developed a method to establish large animal models of GERD using the endoscopic technique. Animal models provide a comprehensive platform for the study of the function and anatomy of the esophagus and stomach, and provides better basis for future studies of the endoscopic treatment of GERD.
Methods
Experimental materials
Olympus GIF-Q260 host (Olympus Corporation, Japan), Olympus GIT-Q260J gastroscope and graphic system (Olympus Corporation, Japan), Olympus UES-30 high frequency electric transmitter (Olympus Corporation, Japan), C-arm X-ray machine (GE, USA), camera (Canon, Japan), mucosal needle (Medwork, USA), and Olympus heat hemostatic forceps and triangular knives (Olympus, Japan).
Experimental animals
In this study, six fresh swine carcasses were used (male or female). The body weight of these carcasses ranged between 35.0–40.0 kg, with an average of 37.14±2.86 kg. No upper gastrointestinal endoscopy training was carried out, which is in accordance to the regulations of the animal experiment.
Experimental animals and processing
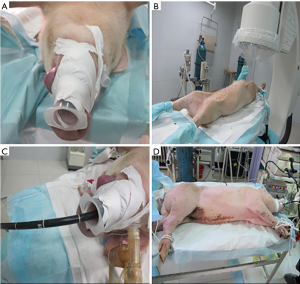
Six fresh swine carcasses were used. Before surgery, a self-made endoscopic pad (a 50 mL syringe was used, the piston was pulled, and the end that was connected to the needle was cut off) was placed, and was fixed in the oral cavity of the carcasses using medical tape (Figure 1).
Surgical procedures
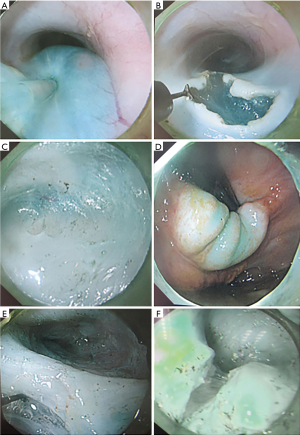
(I) The swine was placed in the supine position. The upper part of the body was raised by 30 degrees and fixed on the contrast bed; (II) esophageal angiography was carried out with 20% meglumine through the gastroscopic channel, and the diameters of the esophagus 5 cm above the dentate line and cardiac orifice were measured; (III) the swine was placed in the left lying position and fixed on the operation table; (IV) a submucosal injection of methylene blue saline solution (1:10,000) was given at the site 10 cm above the dentate line and the site was lifted; (V) an inverted T-shaped incision of 2 cm in length was performed on the mucosa using a triangular knife, the endoscope was inserted through the incision into the submucosal space, and a tunnel to the site 3 cm under the cardia was established; (VI) with the help of a triangular knife, the whole layer of the muscularis propria was cut down from 8 cm above the cardia to 3 cm under the cardia (Figure 2); (VII) the swine was fixed on the contrast bed in the supine position with the upper part raised by 30 degrees, and the diameter of the esophagus 5 cm above the dentate line and cardiac orifice was measured again.
Statistical analysis
Experimental data were analyzed using SPSS 17.0 statistical software. The diameters in these two groups were expressed as mean ± standard deviation (SD). Data obtained at the same site were compared using independent sample t-test. P<0.05 was considered statistically significant.
Results
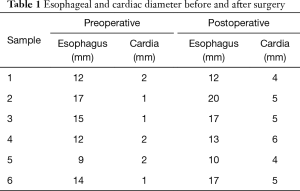
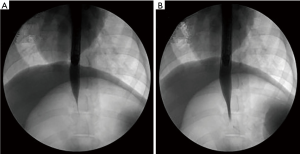
Comparison of esophageal and cardiac diameters before and after surgery (Tables 1,2): a full thickness incision was made on the muscularis propria at the site 5 cm above the dentate line after tunneling. Before and after surgery the diameters of the esophagus 5 cm above the dentate line and cardiac orifice were measured by X-ray radiography and compared (Figure 3). The diameters of esophagus and cardia were 13.167±2.787 and 1.167±0.683 at 5 cm on the preoperative dentate line, and 14.833±3.764 and 4.833±0.753 respectively postoperative. There was no significant change in esophageal diameter at 5 cm on the dentate line before and after operation. P>0.05, was not statistically significant. Cardiac mouth was significantly relaxed after surgery, contrast agent through the smooth. The difference in diameter was statistically significant (P<0.05).
Full table

Full table
Discussion
GERD is a common disease of the digestive system that has various symptoms, extremely easily relapses, and is a refractory chronic disease (7,8). Current treatments include medical, surgical and interventional treatments; but clinical efficacy remains limited (9-11). In order to more scientifically study the mechanism of the occurrence and development of GERD, or to develop new treatment methods, more and more scholars have been involved in related animal experiments. Since the cause and mechanism of this disease remains unknown, it has become a difficulty in related scientific experiments to establish a reasonable and accepted animal model. These methods can be classified into two categories: esophagitis induced by reflux caused by changes in the gastrointestinal structure, and esophagitis induced by exogenous acid filling (12). With the continuous development of digestive endoscopic technology, the application range of digestive endoscopic treatment has also become more extensive. In 2007, the first description of a peroral endoscopic myotomy (POEM) on an animal model was done by a group of North Americans for achalasia (13). In 2009, En-Qiang Linghu, a professor, presented a special report on “Endoscopic submucosal tunnel dissection (ESTD) treatment of lesions in esophageal ring” at the Beijing Digestive Endoscopy Conference, which was named endoscopic tunneling technology (14). In 2010, Inoue, a Japanese scholar (15), reported the technology, POEM. In recent years, the application of POEM treatment for achalasia has annually increased due to its relative satisfactory efficacy and overall safety (16). In follow-ups after POEM, mild reflux symptoms occurred in few patients. In this study, we were inspired from these reports, and proposed whether the tunnel technology can be used to establish a large animal model of gastroesophageal reflux.
In general, animal models help more widely and objectively evaluate various therapeutic methods compared with clinical studies. In the 1970s, Bremner et al. (17) carried out gastroesophagectomy through the removal of LES, in order to establish the first GERD animal model. Then, the animal model established by cardiomyotomy was extensive applied. In recent years, through constant experimental studies, according to the anatomical characteristics of different animals, McMahon et al. (18), Gawad et al. (19) and Poorkhalkali et al. (20) respectively carried out surgical incisions of the LES of dogs, swines and cats. Hence, effective and reliable GERD animal models were established.
A number of animal models have been attempted in the past, but they did not have endoscopic tunneling technology. In the present study, the model of gastroesophageal reflux was established by endoscopically establishing a submucosal tunnel to the cardia to damage the LES. The LES is a physiological sphincter at the junction of the esophagus and stomach. This area is a relatively high pressure area that can prevent the reflux of the stomach and duodenal contents into the esophagus. The gastroesophageal junction of a swine is very similar to that of humans. The surgical incision of LES reduces the pressure of the lower esophageal high pressure zone and damages its normal physiological function, thereby leading to gastroesophageal reflux. Angiography before surgery revealed that the gastroesophageal junction is tightly closed. Furthermore, after incision of the lower esophagus and sphincter, radiography revealed that gastroesophageal junction was obvious relaxed; and enabled the contrast agent to smoothly travel through. We will also conduct further studies on living swines, measure the pressure of the gastroesophageal junction, and perform 24-hour PH monitoring, in order to more objectively evaluate the establishment of the gastroesophageal animal model. Through our previous animal experiments, a large animal model of gastroesophageal reflux was established using the endoscopic tunneling technology, providing better basis for future studies of GERD.
Conclusions
Our animal experiments showed that it is a minimally invasive and mature technology of establishing GERD animal models by using the per-oral endoscopic tunneling technique, and might be a new method to establishing GERD large animal models, and can provide animal models which are more close to human anatomy for GERD basis studies and treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Principles of Laboratory Animal Care’ (NIH Publication Vol 25, No. 28 revised 1996) were followed, as well as specific national laws (e.g., the current version of the German Law on the Protection of Animals) where applicable. The animal study protocol had been approved by the Medical Ethics Committee of Inner Mongolia Medical University. The animal experiments were performed in accordance with the ‘Guide for the Care and Use of Laboratory Animals’.
References
- Eisen G. The epidemiology of gastroesophageal reflux disease: what we know and what we need to know. Am J Gastroenterol 2001;96:S16-8. [Crossref] [PubMed]
- Conio M, Filiberti R, Blanchi S, et al. Risk factors for Barrett’s esophagus: a case-control study. Int J Cancer 2002;97:225-9. [Crossref] [PubMed]
- Arts J, Tack J, Galmiche JP. Endoscopic antireflux procedures. Gut 2004;53:1207-14. [Crossref] [PubMed]
- Chen D, Barber C, McLoughlin P, et al. Systematic review of endoscopic treatments for gastro-oesophageal reflux disease. Br J Surg 2009;96:128-36. [Crossref] [PubMed]
- Lagergren J, Bergström R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825-31. [Crossref] [PubMed]
- Katz PO, Zavala S. Proton pump inhibitors in the management of GERD. J Gastrointest Surg 2010;14:S62-6. [Crossref] [PubMed]
- Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global, evidence-based consensus paper. Z Gastroenterol 2007;45:1125-40. [Crossref] [PubMed]
- Fass R, Ofman JJ. Gastroesophageal reflux disease--should we adopt a new conceptual framework? Am J Gastroenterol 2002;97:1901-9. [PubMed]
- Fornari F, Sifrim D. Diagnostic options for patients with refractory GERD. Curr Gastroenterol Rep 2008;10:283-8. [Crossref] [PubMed]
- Richter JE. How to manage refractory GERD. Nat Clin Pract Gastroenterol Hepatol 2007;4:658-64. [Crossref] [PubMed]
- Dean BB, Gano AD Jr, Knight K, et al. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin Gastroenterol Hepatol 2004;2:656-64. [Crossref] [PubMed]
- Kadirkamanathan SS, Yazaki E, Evans DF, et al. An ambulant porcine model of acid reflux used to evaluate endoscopic gastroplasty. Gut 1999;44:782-8. [Crossref] [PubMed]
- Pasricha PJ, Hawari R, Ahmed I, et al. Submucosal endoscopic esophageal myotomy: a novel experimental approach for the treatment of achalasia. Endoscopy 2007;39:761-4. [Crossref] [PubMed]
- Linghu EQ. Create and prospect of tunnel technology. Chin J Laparoscopic Sirgery 2011;4:326-7.
- Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy 2010;42:265-71. [Crossref] [PubMed]
- Costamagna G, Marchese M, Familiari P, et al. Peroral endoscopic myotomy (POEM) for oesophageal achalasia: preliminary results in humans. Dig Liver Dis 2012;44:827-32. [Crossref] [PubMed]
- Bremner CG, Lynch VP, Ellis FH Jr. Barrett's esophagus: congenital or acquired? An experimental study of esophageal mucosal regeneration in the dog. Surgery 1970;68:209-16. [PubMed]
- McMahon RL, Ali A, Chekan EG, et al. A canine model of gastroesophageal reflux disease (GERD). Surg Endosc 2002;16:67-74. [Crossref] [PubMed]
- Gawad KA, Wachowiak R, Rempf C, et al. Ambulatory long-term pH monitoring in pigs. Surg Endosc 2003;17:1556-60. [Crossref] [PubMed]
- Poorkhalkali N, Rich HG, Jacobson I, et al. Chronic oesophagitis in the cat. Scand J Gastroenterol 2001;36:904-9. [Crossref] [PubMed]


