Adult veno-arterial extracorporeal life support
Introduction
The 1940’s held the development of the artificial kidney by Dr. Willem Kolff. Interestingly, he was able to recognize that blood could be oxygenated as it passed through cellophane chambers. Dr. Gibbon later applied this property to the heart-lung machine in the 1950’s. With the 1960’s, the use of bubble oxygenators fell out of favor for membrane oxygenators in cardiopulmonary bypass. The method of oxygenation has evolved over the decades. Initially, “filmers” were used where blood was thinly spread out into vertical screens promoted oxygenation. Next, “bubblers” were used where blood in containers was mixed with bubbles of oxygen. Today, “membranes” consisting of hollow fibers allow blood to travel through an innumerable amount of thin tubes bathed in oxygen dense solution. Extracorporeal membrane oxygenation (ECMO) was in its infancy in the 1970s. At that time, the indication, applications, and outcomes remained relatively unknown. This led to large collaborative study involving nine hospitals sponsored by the National Heart, Lung, and Blood institute to research extracorporeal life support (ECLS) (1). This study found no difference in survival in adult patients with pulmonary insufficiency who had underwent ECMO therapy as compared to those treated with medical management. Unfortunately there were several problems associated with this study. First, many patients were entered into the study when lung damage had reached irreversible levels. Second, high-pressure ventilator support was continued in many patients thereby perpetuating the damage to lung parenchyma. Other studies in this decade showed similar results.
The 1970’s marked an evolution for ECMO. Baffes et al. successfully applied ECMO to support infants during palliative congenital cardiac procedures (2). Shortly after, Barlett and associates were the first to successfully use ECMO as a rescue therapy in term infants with severe, acute, but reversible respiratory failure (3). The use of ECLS for this indication cemented its utility in being able to sustain life. To date, many medical centers are equipped to provide ECLS to morbidly ill patients with potentially reversible cardiopulmonary derangements.
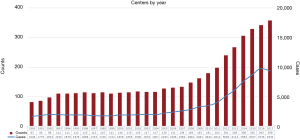
Currently, two variations of ECLS exist. Veno-arterial ECMO (VA-ECMO) is utilized in patients with cardiopulmonary failure, while Veno-venous ECMO (VV-ECMO) is meant for respiratory failure in patients with preserved cardiac function. ECLS temporarily takes over the function of the cardiopulmonary system resulting in the potential to rescue morbidly ill patients. April of 2009 marked the outbreak of pandemic proportions. The H1N1 influenza strain put children and the elderly at significant risk of death from respiratory failure. Accordingly, the use of VV-ECMO from 2009 onward rapidly increased with relatively positive results (Figure 1) (4). Zangrillo et al. conducted a systematic review of the literature including eight studies of patients with severe acute lung injury secondary to H1N1 influenza and found that 266 of 1,357 patients (20%) were placed on ECMO (5). The median ECMO duration was 10 days with an overall in-hospital mortality of 28% (95% CI: 18–37%). This study demonstrated the feasibility of ECMO therapy in acute lung injury from H1N1. They also found that predictors of mortality included length of stay prior to ECMO initiation as well as mean arterial pressure. This is also seen when evaluating of the 2011 ESLO International H1N1 Report, where there was a higher mortality rate in patients requiring ECLS for ≥7 days (6).
ECLS is a powerful tool but fraught with possible complications. Additionally, there remains much room for research into the predictors of successful ECLS therapy. Ethics, risk-benefit analysis, and cost-burden debates are an integral part of the assessment period prior to implementing ECLS in any patient and has become relevant as ECLS therapy becomes more widespread as a means to prolong life in patients with reversible cardiorespiratory failure (7).
Indications and patient selection
Indications for initiation of VA-ECMO are guided by the principle of medically refractory cardiogenic shock. As this therapy has increased in popularity, various algorithms have been developed for treatment of refractory cardiopulmonary failure. The Ohio State University Wexner Medical Center protocol defines as a systolic blood pressure (SBP) of <90 mmHg or mean arterial pressure <65 mmHg for more than 30 minutes, in addition to a need for inotropes, vasopressors or mechanical circulatory support to maintain an SBP >90 mmHg (REF). Evidence of decreased organ perfusion must also be present, such as: persistent lactic acidosis, oliguria, altered mental status, acute renal injury, elevated liver enzymes, pulmonary edema and/or hypoxia. A persistent Cardiac Index <1.9 L/m2 along with high doses of inotropes with hypotension despite adequate fluid resuscitation further confirms the presence of cardiogenic shock. In our experience, the patient populations frequently evaluated for ECLS include those with decompensated heart failure, acute coronary syndrome, peri-partum cardiomyopathy, fulminant myocarditis, and cardiac allograft failure following heart transplantation. Other indications include emergent VA-ECMO [also known as (extracorporeal cardiopulmonary resuscitation) eCPR] for those in cardiac arrest and those in acute respiratory failure with cardiovascular collapse. Some less common indications include anaphylaxis, hypothermia, pulmonary embolism, peripartum cardiomyopathy, and drug overdose (8).
While there are no true, absolute contraindications to the initiation of VA-ECMO, consideration of potential futility is a must (7,8). Those patients with advanced malignancy, coagulopathies, intracranial hemorrhage, age >80 years or any condition that relays a limited life expectancy or high likelihood of failure to separate from ECLS should be evaluated critically and in multidisciplinary fashion when able—preferably prior to support initiation of ECLS.
Technique and implementation
The VA-ECMO circuit consists of a closed system that closely mirrors that of a cardiopulmonary bypass circuit (Figure 2). Essentially, a pump (with or without a heat exchanger), a membrane oxygenator, cannulas for venous drainage and arterial outflow, controller unit, and tubing make up the components of the ECMO circuit. Several commercially available pumps of various designs may be used, most of which are centrifugal by design with flow rates capable of greater than 8 Liters per minute. The Cardiohelp system® (Maquet, Rastatt, Germany) recently became the first commercially available portable device specifically designed for ECLS applications and has since become FDA approved for use in the United States.
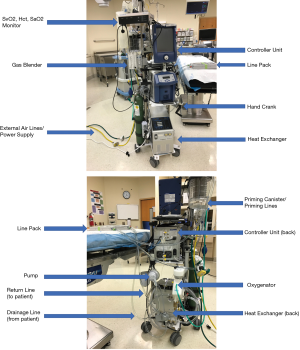
The drainage cannula is typically a large diameter cannula (21–25 French) inserted into the venous system of the patient. Depending on insertion site, the cannula may be of single or multi-drainage holes to meet the demands of a specific scenario. The return cannula delivers blood from the pump and circuit to the patient and is inserted into the arterial system. These cannulas are typically smaller (15–19 French) in diameter and shorter than the venous cannula. Of note, even though current pump systems are capable of flow rates that exceed average cardiac output in even the largest adults, flow is usually limited by the ability to adequately drain the venous system.
Ultimately, cannulation strategies vary depending upon the clinical scenario. In post-cardiotomy patients, typically those patients that fail to wean from cardiopulmonary bypass, or in cases of emergent sternal re-entry following cardiac surgery, central cannulation represents the simplest and most expeditious option. Similar to cannulation for cardiopulmonary bypass, two purse-string sutures within the aortic media are placed to accommodate the chosen cannula diameter into the ascending aorta. An aortotomy is created sharply via a single stab incision with care taken not to injure the back wall of the aorta or dissect the vessel. The arterial cannula is inserted with similar care and secured using Rumel tourniquets on the purse-string sutures. The tourniquets are then secured to the cannula with a free suture ties. The cannula is de-aired in standard fashion and clamped.
The venous cannula, when centrally inserted, typically accesses the right atrium. A single purse-string is placed along the right atrial appendage. The right atrium is then accessed using a single stab incision with care taken to avoid the lateral and back walls of the atrium and the atrioventricular groove. The venous cannula is then inserted and purse-string secured in the standard fashion. The cannula is de-aired and clamped.
Next, in close communication with the perfusion specialist, drainage and return lines from the circuit are clamped and cut to length. The arterial and venous cannulas are then secured the return and drainage lines respectively after being completely de-aired. ECLS is then initiated by slowly increasing the pump speed until adequate flow is achieved with appropriate drainage and line pressures.
In patients in whom the chest is not already open, peripheral femoral access is typically proves the most expeditious. Percutaneous approaches can be used for both arterial and venous access, with or without ultrasound guidance depending upon availability (9). Open cut-down can also be employed owing to applicable scenarios and body habitus. As with any peripheral vascular access scenario, care must be taken during vessel dilation and subsequent Seldinger’s technique of cannulation to avoid damage to the vasculature. Given the large cannula size, particularly of a multi-staged drainage cannula, complications can arise including retroperitoneal hematomas, groin hematomas, or vessel dissection. Once the cannulas are appropriately positioned, they are secured at skin level, clamped, de-aired and attached to the circuit in a similar manner as in central cannulation. Post-procedure abdominal and chest X-rays are utilized to confirm the venous cannula’s position at the caval-atrial junction. Appropriate positioning of the arterial cannula is continually verified through following circuit flow trends, as an inability to maintain adequate flow or development of high line pressures may indicate malpositioning. Also, in our institution, we routinely place a smaller, armored, 7-French distal perfusion cannula in an antegrade fashion into the superficial femoral artery to reduce the risk of limb ischemia. This distal perfusion cannula is then connected to the arterial cannula with an anticoagulant and low-dose nitroglycerin to reduce the risk of limb malperfusion.
Alternative methods for peripheral cannulation are also possible. Venous cannulation via the internal jugular vein can be utilized in cases of inferior caval obstruction or interruption, exclusively if needed, but most often when combined with femoral drainage to ensure adequate drainage. Arterial cannulation may also be accomplished by using an 8 mm graft sewn end-to-side onto either the femoral or axillary artery. This technique avoids the need for additional distal perfusion catheters but can lead to over circulation depending on the distal vessel size and resistance to blood flow. Given the high incidence of vascular complications we suggest using this technique whenever possible.
Once cannulation is effective and support initiated, the adequacy of flows and drainage are verified. Cardiac decompression must be verified and may be augmented by the use of left ventricular (placed either directly/apically or via a pulmonary vein) or pulmonary artery vents that can then be lined into the venous drainage side of the circuit. All cannulas are then secured in several places and their positions marked to prevent malpositioning.
Management
Circuit management consists of a vigilant multidisciplinary monitoring of the ECLS patient. Nursing, perfusion specialists, intensivists, and surgeons must all be aware of changes and trends in the patient’s hemodynamics and circuit components. Appropriate radiographs should be ordered to verify cannulas position as soon as able. This is particular important following patient transport from the operative suite to the intensive care unit. The cannulas’ position should also be verified at skin level and documented routinely. Line pressures should also be documented and trended on an hourly basis, as slowly rising trends could indicate issues such as thrombosis or cannula malpositioning. Blood gases should be measured and trended as well, in addition to visually inspecting the oxygenator for signs of clot formation. Flows are also trended, as fluctuations in such could represent lack of adequate drainage or hypovolemia. Additionally, monitoring the patient’s native cardiac function must also be carried out. As native cardiac output improves as the patient recovers, competing native flow and circuit flow can actually inhibit recovery, prolong recovery, and impede separation from ECLS. In it most severe form, this may even lead to differential oxygenation between the upper and lower parts of the body in the setting of femoral arterial cannulation with deoxygenation blood from persistently depressed native lung function continuing to be circulated to the upper torso with resultant hypoxemia (harlequin syndrome).
Anticoagulation is begun with a bolus of heparin with the aim of achieving an activated clotting time of >220 seconds. This is done ideally prior to cannulation and clamping required for de-airing maneuvers. Activated partial thromboplastin (aPTT) values are followed in the intensive care unit with a goal of maintaining those between 60 and 80 seconds. In patients with heparin-induced thrombocytopenia, other agents may be used, with bivalirudin being the most common choice. In general, and particularly of note when weaning support, lower flow rates increase the risk of thrombus formation and anticoagulation levels should be scrutinized.
Mitigation of any complications that arise is also an important part of circuit management. Oxygenators that become burdened by thrombus formation need to be exchanged. Air entrainment into the system can be a devastating complication that may require a complete circuit change out if appropriate de-airing cannot be accomplished in a timely fashion. Inadvertent decannulation is also a major problem, as patient instability, location in the intensive care unit, and questionable immediate-availability of surgical expertise are barriers to a successful outcome. In this situation, open re-exploration of the cannulation sites in the operating room is ideal, with full cardiopulmonary bypass capability at the ready, particularly in situations that require mitigation of circuit air. It is also prudent to have a backup and primed ECMO circuit at the ready for such catastrophic situations, if logistics allow.
Outcomes
The body of literature examining outcomes of ECLS has been steadily growing ever since its implementation. The Extracorporeal Life Support Organization (ELSO) registry has determined that 56% percent of adult patients survived VA-ECLS after refractory cardiogenic shock while 40% of adults patients survived VA-ECLS in the setting of cardiopulmonary resuscitation (4). Moreover, these survival figures have increased significantly over the past three decades. The survival rates to discharge for the two groups described above were 41% and 30%, respectively (4). A single institution study by Truby et al. found a 39% rate of survival until discharge along with 45% 30-day survival. Myocardial recovery was shown in 80% of surviving patients and a significant number of patients were able to be transitioned to a more durable device (10). Elderly status and indication for VA-ECMO were both found to be powerful predictors of mortality among the cohort studied. In 2016, a large German network published similar survival figures compared with those above in patients undergoing VA-ECLS for indications other than post-cardiotomy shock (11). In addition, this study emphasized the potential benefits of rapid initiation of ECLS in a regional setting with subsequent transfer to a tertiary care center.
While most studies regarding VA ECLS involve cohorts of patients who are hospitalized at the time of cardiogenic shock ECMO initiation for ventricular tachycardia or ventricular fibrillation arrest with eCPR has been gaining evidence, particularly over the last decade. This strategy involves direct transfer of patients with non-perfusing ventricular tachycardia and ventricular fibrillation for ECLS initiation followed by emergent cardiac catheterization. If patients are not found to have obstructive coronary artery disease Electrophysiology consultation is requested for management of primary arrhythmia. Johnson and colleagues published a small study of 26 patients who suffered cardiac arrest either in or out of the hospital. Four of these patients survived and three were neurologically intact at the time of discharge (12). In a 2011 review of Japanese eCPR literature survival rate until discharge was found to be 26%. The rate of full recovery amongst surviving patients was 48%, however, 37% of surviving patients were deemed to be in a “vegetative state” (13). Another study comparing eCPR to conventional CPR for witnessed cardiac arrest showed significantly improved outcomes with little to no neurologic deficit in the VA-ECMO cohort. This cohort also showed a 28% 6-month survival with little to no neurologic deficit (14).
Outcomes after ECLS initiation for reversible insults like fulminant myocarditis are better than those associated with post-cardiotomy shock, and acute myocardial infarction. In fact, one multicenter analysis showed a 76% rate of weaning from VA-ECMO along with a 72% survival until discharge in these patients (15). In the setting of postcardiotomy shock the survival rate to discharge is likely closer to 30% (16).
Numerous complications resulting from ECLS have been described. The most common of these can be grouped into multi-organ failure, vascular complications, as well as bleeding. Hemorrhage can occur at various sites in patients supported with ECMO. These include cannulation sites, the gastrointestinal tract, cerebrovascular, and prior operative sites. Consumptive coagulopathy as well as pharmacologic anticoagulation seem to play a significant role in these complications. The rate of significant bleeding during VA-ECLS therapy has been estimated at 40% (17). Lower extremity complications due to malperfusion include lower extremity ischemia (15–20%), necessity for lower extremity fasciotomy (10%). These complications have been linked to a 5% rate of limb loss (17). Acute kidney injury occurs in 55% percent of patients supported with VA-ECLS; approximately 45% of these patients require renal replacement therapy. Neurologic complications occur in 10–15% of patients with stroke occurring in 6%.
Discussion
VA-ECLS is a powerful tool, which can be used to support patients with end-stage cardiopulmonary failure. Since its inception in the mid-19th century numerous patients with cardiopulmonary failure have been rescued from certain death. However, its implementation has been associated with various complications and concerns. These concerns have come up frequently as the use of VA-ECLS has been expanded, particularly over the last decade.
The mortality rate of patients treated with VA-ECMO is undoubtedly high. This fact alone should not discourage its use. Mortality rates of patients with end stage cardiopulmonary failure treated medically are undoubtedly higher than those of patients treated with VA-ECMO. This difference is augmented in patients with reversible causes of heart failure due to myocarditis and/or ventricular arrhythmias. Thus, while the mortality associated with VA-ECLS is high, there are numerous patients who are rescued by its use every year. Patients with reversible causes of myocarditis in fact have survival rates of up to 70%. While survival of patients supported by VA-ECLS is established and significant, morbidity associated with this therapy remains an issue that must be considered prior to its initiation.
VA-ECMO is associated with a relatively high rate of complications. However, the moribund state of patients treated with it puts them at quite a significant risk of having similar, if not worse, end organ complications without its use. Despite this, physicians must always consider the risk-benefit ratio of placing patients on ECMO. Future technological advancements with undoubtedly reduce the rates of these various complications.
Most importantly, institutions must have an algorithmic approach to initiating VA-ECMO support. Standardization of this process leads to improved outcomes, as well as understanding between various hospital teams as far as which patients are candidates for this therapy. Assessing the reversibility of cardiorespiratory failure remains the most important aspect of these algorithms. ECLS support for irreversible causes raises difficult ethical questions regarding the therapy. This is particularly true in younger patients who often undergo more aggressive attempts at rescue.
In conclusion, VA-ECLS has given physicians the ability to rescue patients from some of the most devastating forms of cardiac and cardiorespiratory failure. This therapy, however, does not represent a panacea by any means. Indeed, it is associated with various morbidities and a high rate of mortality particularly in patients suffering from post-cardiotomy shock. For these reasons, a risk benefit analysis should be carefully considered prior to initiation. An algorithmic approach to its usage should also be adopted in order to standardize its implementation.
Future directions
As technology and outcomes improve great potential lies in educating various first responders about ECMO and its various indications and complications. Early initiation of this therapy with the cooperation of emergency medicine physicians, cardiologists, and critical care physicians will further improve outcomes of ECLS therapy. Complications are still quite common in ECLS. The presence of spontaneous echo contrast seen on echocardiography in instances where red blood cells aggregate during low flow states or areas of stasis places patients at risk for clot formation. A study by Unai et al. reported 22% of their study patients had this finding (18). This group of patients had a significantly higher risk of intra-cardiac thrombus formation and stroke. Further research into pulsatile and antegrade flow may be important in myocardial recovery and to prevent stasis (18,19). Heart failure management continues to evolve. Patients are able to survive longer and with improved quality of life on left ventricular assist devices. ECLS may prove to be advantageous in “off-pump” implantation of these devices and warrant further research (20). Additionally, there is room for research and investigation in the construction of ECLS circuit components as well as flow physiology. The balance between antegrade and retrograde flow give way to the watershed phenomenon where there is differential perfusion of the upper body and brain for example. Therefore, alteration of the ECMO speed alone to “shift” the watershed zone may not be sufficient. Additional research into left ventricular unloading, medical management, and medical optimization is needed (21). Clot formation can occur for a variety of reasons. The contact surface between blood and machine/circuit is critical. Development of nitric oxide releasing polymers embedded into the circuit tubing may reduce thrombogenicity (22). Moreover, the applications of ECLS may be expanded into the realm of transplantation, particular in donation following cardiac death (23). This potentially increases the donor pool, but remains highly debated from an ethical and judicial standpoint (24). ECLS is a powerful tool in the surgeon’s armamentarium. The field of ECLS continues to improve over time and presents as an exciting opportunity for surgeon-scientists to discover and refine.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979;242:2193-6. [Crossref] [PubMed]
- Baffes TG, Fridman JL, Bicoff JP, et al. Extracorporeal circulation for support of palliative cardiac surgery in infants. Ann Thorac Surg 1970;10:354-63. [Crossref] [PubMed]
- Bartlett RH, Gazzaniga AB, Jefferies MR, et al. Extracorporeal membrane oxygenation (ECMO) cardiopulmonary support in infancy. Trans Am Soc Artif Intern Organs 1976;22:80-93. [PubMed]
- Extracorporeal Life Support Organization. ESLO International Registry Report. Ann Arbor, MI, USA, 2017.
- Zangrillo A, Biondi-Zoccai G, Landoni G, et al. Extracorporeal membrane oxygenation (ECMO) in patients with H1N1 influenza infection: a systematic review and meta-analysis including 8 studies and 266 patients receiving ECMO. Crit Care 2013;17:R30. [Crossref] [PubMed]
- Extracorporeal Life Support Organization. ESLO International H1N1 Registry Report. Ann Arbor, MI, USA, 2011.
- Shah AS. Mobile extracorporeal membrane oxygenation: Lead, follow, or get out of the way. J Thorac Cardiovasc Surg 2017;153:1369-70. [Crossref] [PubMed]
- Kilic A, Shukrallah BN, Kilic A, et al. Initiation and management of adult veno-arterial extracorporeal life support. Ann Transl Med 2017;5:67. [Crossref] [PubMed]
- Napp LC, Kühn C, Hoeper MM, et al. Cannulation strategies for percutaneous extracorporeal membrane oxygenation in adults. Clin Res Cardiol 2016;105:283-96. [Crossref] [PubMed]
- Truby L, Mundy L, Kalesan B, et al. Contemporary Outcomes of Venoarterial Extracorporeal Membrane Oxygenation for Refractory Cardiogenic Shock at a Large Tertiary Care Center. ASAIO J 2015;61:403-9. [Crossref] [PubMed]
- Aubin H, Petrov G, Dalyanoglu H, et al. A Suprainstitutional Network for Remote Extracorporeal Life Support: A Retrospective Cohort Study. JACC Heart Fail 2016;4:698-708. [Crossref] [PubMed]
- Johnson NJ, Acker M, Hsu CH, et al. Extracorporeal life support as rescue strategy for out-of-hospital and emergency department cardiac arrest. Resuscitation 2014;85:1527-32. [Crossref] [PubMed]
- Morimura N, Sakamoto T, Nagao K, et al. Extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest: A review of the Japanese literature. Resuscitation 2011;82:10-4. [Crossref] [PubMed]
- Shin TG, Choi JH, Jo IJ, et al. Extracorporeal cardiopulmonary resuscitation in patients with inhospital cardiac arrest: A comparison with conventional cardiopulmonary resuscitation. Crit Care Med 2011;39:1-7. [Crossref] [PubMed]
- Lorusso R, Centofanti P, Gelsomino S, et al. Venoarterial Extracorporeal Membrane Oxygenation for Acute Fulminant Myocarditis in Adult Patients: A 5-Year Multi-Institutional Experience. Ann Thorac Surg 2016;101:919-26. [Crossref] [PubMed]
- Elsharkawy HA, Li L, Esa WA, et al. Outcome in patients who require venoarterial extracorporeal membrane oxygenation support after cardiac surgery. J Cardiothorac Vasc Anesth 2010;24:946-51. [Crossref] [PubMed]
- Cheng R, Hachamovitch R, Kittleson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg 2014;97:610-6. [Crossref] [PubMed]
- Unai S, Nguyen ML, Tanaka D, et al. Clinical Significance of Spontaneous Echo Contrast on Extracorporeal Membrane Oxygenation. Ann Thorac Surg 2017;103:773-8. [Crossref] [PubMed]
- Napp LC, Tongers J, Schmitto JD, et al. Venoarterial Extracorporeal Membrane Oxygenation: Lower Speed, and You May Be Faster. Ann Thorac Surg 2017;104:724-5. [Crossref] [PubMed]
- Rojas SV, Haverich A, Schmitto JD. Off-pump versus on-pump left ventricular assist device exchange. Artif Organs 2014;38:992. [Crossref] [PubMed]
- Napp LC, Schmitto JD, Tongers J, et al. The short- and long-term risks of venoarterial extracorporeal membrane oxygenation watershed. Eur J Cardiothorac Surg 2018;53:894. [Crossref] [PubMed]
- Major TC, Handa H, Annich GM, et al. Development and hemocompatibility testing of nitric oxide releasing polymers using a rabbit model of thrombogenicity. J Biomater Appl 2014;29:479-501. [Crossref] [PubMed]
- Magliocca JF, Magee JC, Rowe SA, et al. Extracorporeal support for organ donation after cardiac death effectively expands the donor pool. J Trauma 2005;58:1095-101; discussion 1101-2. [Crossref] [PubMed]
- Veatch RM. Donating hearts after cardiac death--reversing the irreversible. N Engl J Med 2008;359:672-3. [Crossref] [PubMed]


