Robotic mitral valve repair in infective endocarditis
Background
The first robotic mitral valve repair was performed by Carpentier in 1998 using a prototype of the da VinciTM surgical system (Intuitive Surgical, Inc., Sunnyvale, CA, USA) (1), since then the concept of robotic mitral valve surgery became reality, and now robotic mitral surgery is the most common robotic cardiac procedures. Mitral valve repair in degenerative disease is the evidence-based care standard, not only in traditional sternotomy approach but also in robotic surgery. However, in mitral endocarditis the repair become more challenging especially in minimally approach.
Infective endocarditis is a serious disease of the endocardium of the heart and cardiac valves, caused by a variety of infectious agents. Treatment of endocarditis includes prolonged appropriate antimicrobial therapy and in selected cases, cardiac surgery. The techniques in mitral endocarditis repair are much more challenging than degenerative disease not only in standard sternotomy but also in minimal incision. The benefit of minimally invasive incisions is well documented in the literature (2). Several large health care delivery systems have embraced minimally invasive surgical approaches aiming to replicate the “gold standard” results of a trans-sternal cardiac surgery aiming to improve patient acceptance and facilitate earlier referral. With more and more experience in handling robotic surgical system, we applied robotic surgery in mitral endocarditis repair.
Patients
From January 2012 to December 2013, we operated 12 patients with mitral endocarditis operated via robotic assisted in National Taiwan University Hospital were analyzed. Age of them was among 21 to 65 years old, mean 43. Among them eight are operated in active endocarditis phase and four of them had been treated with antibiotics for three weeks. The diagnosis of infective endocarditis was documented on cardiac echogram findings and infection specialist’s judgments. All of them had vegetation on mitral valve and causing severe mitral regurgitation.
Description of operation technique
Anesthesia preparation
Patients are intubated with either a double lumen endotracheal tube or a bronchial blocker to allow for right lung isolation. All patients were put on transesophageal echocardiogram for valve lesion study.
Position
The patient is positioned with the right side up thirty degrees from horizontal. Bilateral femoral area and right neck should be disinfected and wrapped for further peripheral cannulation.
Cardiopulmonary bypass setting
Usually right side femoral artery and femoral vein are used for bypass route. Right side internal jugular vein is also cannulated for drain. Negative pressure system is routinely used in our minimally invasive surgery.
Ports
A 3 cm working port incision is made in the 4th intercostal space anterior to the anterior axillary line (AAL). Camera port is inserted at 4th intercostal space, just around right nipple areola line. Robotic arm trocars are introduced, one in the 5th or 6th intercostal space at the AAL for the right arm, one in the 2nd or 3rd intercostal space anterior to the AAL for the left arm. The dynamic atrial retractor is inserted in the 5th intercostal space three fingerbreadths medial to the nipple (Figure 1). The da VinciTM system is then docked.

Myocardial protection
The ascending aorta is occluded using the Chitwood transthoracic aortic cross clamp, and antegrade crystalloid cold cardioplegia is used to arrest the heart. The preoperative chest computed tomography is routinely used to evaluation the calcification of ascending aorta.
Surgical strategies in mitral endocarditis
Check the lesion and resected the infected area
After arrest, Sondergaard’s groove is dissected, and the entry of the pulmonary veins into the left atrium is identified. A left atriotomy is performed and the dynamic atrial retractor is used to expose the mitral valve. The valve lesion is inspected segmentally (Figure 2A). The infected area should be removed to ensure the curative of infected vegetation (Figure 2B). After all the infected tissue resected, re-check the valve again to decide further treatment plan (Figure 2C).

Evaluate the valve condition
The valve leaflet is checked again to ensure good size for coaptation. If there is any hole in the anterior leaflet, patch repair of the leaflet first. If any chordae lossing, we use CV-4 GoretexTM suture to reconstructe new chords (Figure 2D).
After reconstruction of valve, the annulus is always support with Cosgrove-Edwards band for all of them (Figure 3).
Antibiotics and medical treatment
All patients receive a 4-6-week antibiotics course treatment. The C reactive protein is monitored in all of them. Besides no fever, before discharged home, the C reactive protein should be normal in range. Cardiac echography follow is performed in all of them before discharged home.
Result
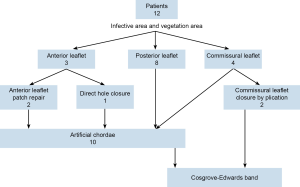
Among January 2012 to December 2013, 12 patients underwent robotic mitral repair for their mitral endocarditis. Preoperative, all of them have severe mitral regurgitation and vegetation detectable by cardiac echography. The vegetation involves anterior leaflet in 3, posterior leaflet in 8 and commissural leaflet in 4 (Figure 3).
Mean cardiopulmonary bypass time is 124 minutes and cross clamp time is 89 minutes. There was no stroke and no operation death. Mitral valve repair technique including anterior leaflet patch augmentation in 2, and direct closure of rupture hole on anterior leaflet in one. We closed two commissural leaflets by plication commissural leaflet. Ten of them require artificial chordae to regain the coaptation height (Figure 3).
Mitral valve condition
Valve insufficiency was defined as more than mild grade valve dysfunction on cardiac echography. There was no mitral regurgitation detected immediately after weaning of cardiopulmonary bypass in ten of them, and two with trace to mild mitral regurgitation. All of them got free-from-regurgitation or -stenosis rate was 100% at one-year follow. There was no recurrent fever or vegetation noted on cardiac echography finding.
Discussion
Robotic cardiac surgery is still evolving despite being deployed for over a decade. Iterative advancements in device technology suggest to many of us that tissue telemanipulation and the least invasive methods will become a major part of our surgical field in the future (3). Mitral repair in degenerative disease is described much more than infective endocarditis in the literature and in real practice (3-5). Infective mitral endocarditis is complex, for the variation of disease severity and variety of valve condition. Many surgeons remain so concerned with the complexity and the procedure cost that they will not adopt this platform (3).
The surgical principles applicable in the treatment of infective endocarditis including debride infected tissues and all vegetation then restoration of damaged structures (6). Debride the infected tissue is mandatory in the surgical treatment for infective endocarditis followed by restore of leaflet function and size (7). Anterior leaflet size is important for the good long term coaptation, if the defect over anterior leaflet is big enough to influent the area of anterior leaflet, we will use the patch to augment anterior leaflet size. For the posterior leaflet lesion, in our practice, we favored using artificial chordae to preserve as much leaflet tissue as possible. Based on the above principles and the surgical techniques, described by A. Carpentier (7) in recent years, a high rate of success of such complex mitral valve repair procedures has been attained in mitral valve infective endocarditis, with some centers having a success rate of 75-80% (2,6). In United States, about 25% of mitral valve operations were done using minimally invasive techniques, and that only half of them using robotic mitral surgery (8). There is only few report of repair mitral endocarditis by robotic system. For the complexity of the lesion and surgeons are afraid of adopting robotic system on difficult cases. However, with the benefit of minimally invasive technique become more well known and there should be more and more patients and surgeons prone to robotic assisted mitral operation (4,5). We demonstrated robotic mitral repair in this complex lesion and complicated patient group. The result is good and could apply all mitral repair techniques on mitral repair. With more familiar with the robotic system application, the surgery will become more reliable and the result will be constant.
Conclusions
Although mitral infective endocarditis is complex and difficult to repair, robotic mitral repair in infective endocarditis is feasible. We have no operation mortality and all the patients can free from mitral regurgitation and infection after complete course of treatment. Even in the complex repair group, the cardiopulmonary bypass time is not prolonged and the result is good.
Limitation
The study limitation was that it was single-institution case series and did not have a comparative group to the robotic technique. It exist patient selection bias in disease severity and surgeon’s preference.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Carpentier A, Loulmet D, Aupècle B, et al. Computer assisted open heart surgery. First case operated on with success. C R Acad Sci III 1998;321:437-42. [PubMed]
- Mihaljevic T, Jarrett CM, Gillinov AM, et al. Robotic repair of posterior mitral valve prolapse versus conventional approaches: potential realized. J Thorac Cardiovasc Surg 2011;141:72-80.e1-4.
- Kaneko T, Chitwood WR Jr. Current readings: status of robotic cardiac surgery. Semin Thorac Cardiovasc Surg 2013;25:165-70. [PubMed]
- Mandal K, Alwair H, Nifong WL, et al. Robotically assisted minimally invasive mitral valve surgery. J Thorac Dis 2013;5:S694-703. [PubMed]
- Seco M, Cao C, Wilson MK, et al. Systematic review protocol: robotically-assisted minimally invasive mitral valve surgery. Ann Cardiothorac Surg 2013;2:678. [PubMed]
- Zegdi R, Debièche M, Latrémouille C, et al. Long-term results of mitral valve repair in active endocarditis. Circulation 2005;111:2532-6. [PubMed]
- Carpentier A. Cardiac valve surgery--the “French correction”. J Thorac Cardiovasc Surg 1983;86:323-37. [PubMed]
- Nifong LW, Rodriguez E, Chitwood WR Jr. 540 consecutive robotic mitral valve repairs including concomitant atrial fibrillation cryoablation. Ann Thorac Surg 2012;94:38-42; discussion 43. [PubMed]


