Influence of lung resection volume on risk of primary spontaneous pneumothorax recurrence
Introduction
Video-assisted thoracoscopic surgery (VATS) is the standard procedure for the treatment of primary spontaneous pneumothorax (PSP) because it is less invasive, causes less postoperative pain, requires shorter hospital stays, and enables a fast return to work. Thoracoscopic stapled bullectomy reduces operation time and simplifies surgical procedures. However, postoperative recurrence is still concerning, and additional procedures, such as pleurectomy, pleural abrasion, and reinforcement of the staple lines, have been considered (1,2).
Missed bullae has been considered to be the reason for recurrent pneumothorax after VATS, and new bulla formation has been thought to be an important etiology. New bullae are found near staple lines and have been suggested as a reason for pneumothorax recurrence after thoracoscopic bullectomy (1,2). A different tension at the staple line has been suggested to be the cause of bullagenesis, but the exact mechanism of new bulla formation is uncertain.
We hypothesized that different volumes of resected specimens might cause differences in tension for the lung, especially at the staple lines. This study was conducted to evaluate the relationship between postoperative PSP recurrence and the resected volume of apical segment.
Methods
Patients under the age 30 with PSP who had undergone first-time VATS between April 1, 2009 and December 31, 2013 were retrospectively identified. We selected 360 cases of VATS performed in 340 patients for inclusion. Twenty patients had undergone bilateral VATS for bilateral pneumothorax or unilateral pneumothorax with contralateral visible bullae identified by chest computed tomography. Tuberculosis, severe emphysema and catamenial pneumothorax were excluded in this study.
All operations were performed with the patient under general anesthesia with one lung ventilation. Under thoracoscopy bullae were identified, grasped with the endoscopic instruments, and excised with endoscopic staplers. Each lung was resected with a sufficient margin from the bulla. Mechanical pleurodesis or apical pleurectomy were not performed, and fibrin glue or Neoveil (Gunze Co., Ltd., Tokyo, Japan) were used to cover the stapling margin at the individual surgeon’s discretion. The lung was re-inflated after placement of a chest tube in the apex through one of the port sites. In case of incomplete expansion of the lung or the presence of air leakage, a thoracic suction system (−20 cmH2O) was applied.
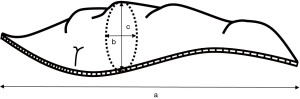
The patients’ electronic medical records, including all operative notes and pathologic reports, were reviewed. The pathologic reports gave the size of the resected specimen as a × b × c (Figure 1). We named the largest dimension “a,” among a, b, and c, as the longest length (cm). “a” was exclusively the length of the staple line. The specimen volume was calculated as a × b × c (cm3). Area (b × c) was used to mean the vertical area against the direction of stapling.
The end point of this study was the first episode of recurrence after first-time VATS for PSP irrespective of management method or the amount of pneumothorax. Long-term follow-up information was obtained by conducting a telephone survey with the patients.
This retrospective study was conducted after permission (OC17RESE0137) was obtained from the Institutional Review Board of Incheon St. Mary’s hospital.
Statistical analysis
Categorical variables are presented as frequencies and percentages. Continuous variables are expressed as the mean ± standard deviation. Continuous variables were compared by using the paired t-test or rank-sum test, and categorical variables were compared by using chi-square or Fisher’s exact test, as appropriate. The Kaplan-Meier method was used to determine the cumulative probabilities of recurrence, along with long-rank tests of significance. The variables included in the multivariate analysis were those detected by univariate models as having a significant association (P<0.05). Cox regression models were used to identify significant risk factors of recurrence. The analyses were performed by using SPSS.
Results
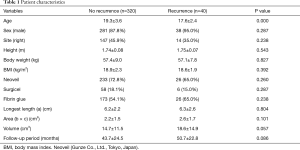
The mean age at the time of operation was 19.1±3.5 (range, 10–30) years. Male patients predominated (269, 74.7%). The maximum follow-up period was 96.1months, with a mean of 44.5±24.4 months. Overall, of the 360 operations, the PSP recurrence rate was 11.1% (40/360).
The mean age was significantly lower in the recurrence group than in the non-recurrence group (17.6±2.4 vs. 19.3±3.6, respectively; P=0.000). Sex, height, body weight, and body mass index were similar between the groups. Reinforcing materials, such as Neoveil (Gunze Co., Ltd., Tokyo, Japan), Surgicel®, and fibrin glue, were used at the discretion of the individual surgeons, and the usage rates were similar. The follow-up periods were longer in the recurrence group than in the non-recurrence group, but the differences was not statistically significant (50.7±22.8 vs. 43.7±24.5, respectively; P=0.086) (Table 1).
Full table
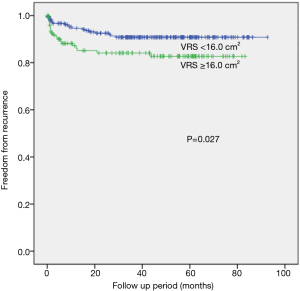
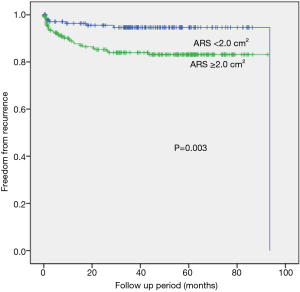
When the resected segment volume cut off value was set at 16 cm3, which is close to the mean resected segment volume value, the ≥16 cm3 group had a significantly higher recurrence rate than those of smaller-volume groups (P=0.027 by the log-rank test) (Figure 2). When we compared the freedom from recurrence rates for groups with different resected specimen vertical areas (cut off value of 2.0 cm2), the larger-area group (≥2.0 cm2) had a statistically significant higher rate of recurrence after VATS than did the smaller-area group (P=0.003 by the log-rank test) (Figure 3).
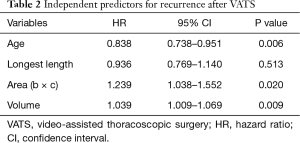
The predictors for recurrence after VATS were identified by multivariate analysis. Age [hazard ratio (HR), 0.083; P=0.006], vertical area of the resected specimen (HR, 1.239; P=0.020), and resected pathology specimen volume (HR, 1.039; P=0.009) were independent risk factors of recurrence (Table 2).
Full table
Discussion
The principle findings of this study were that larger resected specimen volumes and larger vertical section areas were significantly associated with PSP recurrence after VATS.
PSP is usually treated with VATS, which is associated with less invasiveness, reduced postoperative pain, cosmetic benefits, and shorter hospital stays. Although advances in thoracoscopic surgery with staplers have been made, postoperative recurrence remains high (3,4), and it is an important concern of thoracic surgeons. Additional procedures, including parietal pleurectomy, pleural abrasion, and coverage of the staple line with various materials, have been performed to reduce the likelihood of recurrence (1,2), but they increase operation times and cause pain, fever, postoperative bleeding, and infection.
The oversight of bullae and the formation of new bullae have been suggested as etiologies of recurrence after VATS (3). Currently, the primary reason for the high recurrence rate after VATS is considered to be new bulla formation mainly near the staple lines, and the tension on the stapling line has been suggested as the cause of new bulla formation (1,2,5,6). Tsuboshima et al. (5) reported a 29.2% (28/96) rate of postoperative bulla neogenesis, based on bulla identification by high-resolution computed tomography. They explained that the remaining lung after stapled resection has a tendency to return to its original shape; it is prone to over-expansion, and increased tension on the staple line induces new bulla formation. In another report, Muramatsu et al. (7) found that a bullectomy performed with a stapler increased the tension on the staple line during re-inflation, caused deformation of alveolar bronchioles near the staple line and constriction caused by a check valve mechanism, and resulted in a new bulla around the staple line.
Tsuboshima et al. (5,6) hypothesized that a greater extent of lung resection theoretically increased the tension applied to the staple line on the residual lung and that a longer staple line of the residual lung had the more opportunities to cause postoperative bulla neogenesis. Their results showed that a lung weight of ≥1.5 g was a significant risk factor for postoperative bulla neogenesis. Among patients with lung lengths of >4.0 cm, a new bulla developed in 47.9% of such patients. The researchers hypothesized that a greater extent of lung resection increased the tension of the staple line on the residual lung and the longer staple line of the residual lung increased the likelihood of postoperative bulla neogenesis development.
Our study also showed that the resected volume was an important risk factor for recurrence after stapled lung resection. Unlike some previous studies (5,6), we adopted a lung volume based on the three-dimensional volume rather than on lung weight, and our study did not show any relationship between lung length and recurrence. The resected lung has a three-dimensional shape, and the tension of the lung surface is related to the radius of resected lung. As in Tsuboshima’s studies, the tension on the staple line was defined as the sum of individual tensions on all staple lines, and the lung length might be related to bulla neogenesis. However, the vector of individual tension is not in the horizontal direction of staple line but, rather, in the vertical direction of the staple line. Voluminous resection resulted in a round shape and led to greater tension on the staple line than the tensions associated with elliptical and narrow shapes in the vertical section. An increased b × c multiplicative value our study indicated a rounder shape of the vertical area of the resected specimen. An increased vertical area (b × c) indicated a rounder specimen shape and is associated with greater tension than that of an elliptical shape. This increased tension is related to recurrence.
The goals of surgical treatment of pneumothorax are to identify and remove the source of air leakage and achieve pleural symphysis to minimize recurrence of the pneumothorax (8). Absence of pleural symphysis is associated with no formation of pleural adhesions and is related to early or late recurrent pneumothorax. Regardless of stapling length, voluminous resection of the apical segment of the lung leaves a large residual apical space. Absence of pleural symphysis related to a residual apical space increases the risk of recurrence (8).
Remaining air in the airway associated with incomplete lung collapse before stapler firing results in a round shape of the resected lung segment that is associated with recurrence. Therefore, the firing of an endostapler after complete collapse of lung could reduce the tension and perhaps decrease the rate of recurrence.
Our study has limitations. The causes of recurrence after VATS are multifactorial. Besides the neogenesis of bullae around the stapling line, missed bullae and new bullae far away from the staple line can cause recurrence. We did not examine the direct relation between the presence of a new bulla and the volume of the resected lung but instead identified the association between the pathological specimen volume and recurrence. New bulla formation is directly associated with the tension of the resected lung, but recurrence is clinically more important, and recurrence is also related to new bulla formation.
Usually thoracic surgeons select a wide excision up to the normal parenchyma of the lung for complete resection of the bulla, including emphysematous lesion, because surgeons generally believed that wide resection with a sufficient margin is safe, especially based on cancer surgery training. However, our study demonstrated that voluminous resection of the lung was significantly associated with PSP recurrence after VATS. Therefore, we think that both avoidance of deep and broad resection and firing of the stapler only after complete collapse of the lung might reduce the long-term recurrence rates after VATS.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was conducted after permission (OC17RESE0137) was obtained from the Institutional Review Board of Incheon St. Mary’s hospital. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Muramatsu T, Ohmori K, Shimamura M, et al. Staple line reinforcement with fleece-coated fibrin glue (TachoComb) after thoracoscopic bullectomy for the treatment of spontaneous pneumothorax. Surg Today 2007;37:745-9. [Crossref] [PubMed]
- Sakamoto K, Takei H, Nishii T, et al. Staple line coverage with absorbable mesh after thoracoscopic bullectomy for spontaneous pneumothorax. Surg Endosc 2004;18:478-81. [Crossref] [PubMed]
- Ohno K, Miyoshi S, Minami M, et al. Ipsilateral recurrence frequency after video-assisted thoracoscopic surgery for primary spontaneous pneumothorax. Jpn J Thorac Cardiovasc Surg 2000;48:757-60. [Crossref] [PubMed]
- Kim KH, Kim HK, Han JY, et al. Transaxillary minithoracotomy versus video-assisted thoracic surgery for spontaneous pneumothorax. Ann Thorac Surg 1996;61:1510-2. [Crossref] [PubMed]
- Tsuboshima K, Nagata M, Wakahara T, et al. Association between postoperative bulla neogenesis at the staple line and resected lung weight for primary spontaneous pneumothorax: a retrospective study using the inverse-probability of treatment weighted method in patients grouped according to age. J Thorac Dis 2016;8:3676-81. [Crossref] [PubMed]
- Tsuboshima K, Nagata M, Wakahara T, et al. Relationship between postoperative bulla neogenesis at the staple line and the resected lung volume in primary spontaneous pneumothorax. Gen Thorac Cardiovasc Surg 2015;63:572-5. [Crossref] [PubMed]
- Muramatsu T, Nishii T, Takeshita S, et al. Preventing recurrence of spontaneous pneumothorax after thoracoscopic surgery: a review of recent results. Surg Today 2010;40:696-9. [Crossref] [PubMed]
- Gaunt A, Martin-Ucar AE, Beggs L, et al. Residual apical space following surgery for pneumothorax increases the risk of recurrence. Eur J Cardiothorac Surg 2008;34:169-73. [Crossref] [PubMed]