Left atrial appendage exclusion—Where do we stand?
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia and a major cause of morbidity and mortality (1,2). The lifetime risk of AF in men and women over 40 years of age is 1 in 4 (1). It affects 3% to 5% of the population older than 65 years and >13% for those >80 years of age. With an aging population, the prevalence is likely to increase 2.5-fold over the next 50 years (3). Patients with AF have a fivefold higher risk of stroke, which is the number one cause of long-term disability and the third leading cause of death in patients with AF. Over 87% of all strokes are felt to be thromboembolic (1,3). Cardioembolic stroke is the most serious and life threatening potential complication of AF, with an associated mortality up to 30% at 12 months and a 1 in 3 recurrence rate at five years (2). AF is responsible for 25% of all ischemic strokes, occurring in 5% of non-anticoagulated patients every year. In non-valvular AF more than 90% of thrombus accumulation originates in the left atrial appendage (LAA), thus making the LAA an attractive therapeutic target (1-3). Stroke prophylaxis is therefore a socio-economically highly relevant component of management of AF (2).
Traditionally, prophylactic long-term oral anticoagulation (OAC), such as warfarin, is the current method of choice for primary and secondary prevention of thromboembolic events related to AF (1,4). The efficacy of chronic anticoagulation therapy to prevent ischemic strokes in AF is well established. Adjusted-dose warfarin reduces stroke by 60% and death by 25% compared with no antithrombotic treatment. Therefore current guidelines recommend an antithrombotic regimen with warfarin to prevent thromboembolism to all patients with AF and high risk of stroke (3). In contrast to its efficacy, there are many factors that prevent universal use of warfarin in these patients. Firstly, warfarin has a narrow therapeutic window, requires frequent monitoring, has significant drug-to-drug interactions and increases the risk of bleeding. It has significant side-effects, especially in the aged. In some series up to 40% (14-44%) of patients have relative or absolute contraindications to chronic warfarin therapy (1,3).
Consequently, an alternative, effective low-risk therapy is required (4). Novel non-pharmacologic approaches are developed, as a possible treatment strategy for patients at risk for thromboembolic stroke originating from the LAA. LAA exclusion for those patients with contraindications to warfarin and as an alternative to it can be easily performed by either intraoperative excision or obliteration/occlusion using ligation, sutures, or staplers (1,4). Thoracoscopic procedures and percutaneous transcatheter delivery of implantable devices and of the novel Lariat suture delivery device (SentreHEART, Inc., Redwood City, CA, USA) are additional, less invasive methods for LAA exclusion (4). The aim surgical or percutaneous procedures, is complete obliteration of the LAA. However, it can be easily damaged leading to increased postoperative bleeding. Furthermore, its occlusion is often unsuccessful, regardless of the technique used, with a presence of a patent flow or a residual stump in the pouch of the LAA, resulting in an increased risk of late thromboembolic events (4) (Table 1).
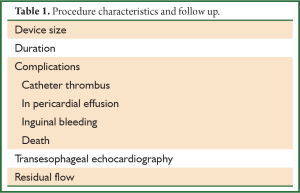
Full table
LAA anatomy and role
The atrial appendages have several unique features. They have a different embryonic origin compared to the atria, as the formation of the two appendages differentiates the morphologically right and left sides of the primary atrium. It is believed to be a remnant of the embryonic left atrium (5). While the atria are smooth-walled, the appendages contain numerous trabeculae (pectinate muscles) forming crypts, resembling the ventricles. This trabeculated blind spot presents a complex and highly variable anatomy, with a long, tubular, often multilobed body extending over the atrioventricular groove and left ventricular surface, and an oval-shaped ostium located between the left ventricle and the left upper pulmonary vein (3). The LAA lies in the left atrioventricular sulcus, overhanging the left circumflex coronary artery and the great cardiac vein; its orifice is typically anterior and inferior to the left superior pulmonary vein, whereas its body is anterior and superior to the left ventricle (1,2). Interestingly, the epicardium on the surface of the atrial appendages is significantly thicker than over the ventricles. In addition, the LAA lies in close epicardial contact to the left ventricle within the confines of the pericardium.
The physiological properties and anatomical relations of the LAA render it ideally suited to function as a decompression chamber during left ventricular systole and during other periods when left atrial pressure is high (3,6). The atrial appendages also function as storage for atrial natriuretic factor (ANF), and perform an important physiologic function regulating the intravascular volume via release of atrial natriuretic peptide. In normal hearts, 30% of the ANF is contained in the LAA. High concentrations of ANF correspond to high activities of the NPPA-gene, which is linked to early development of the heart and to fetal gene reprogramming during heart failure.
There are researches suggesting that LAA plays a role as a reservoir of multiple types of endogenous cardiac progenitor cells (CPCs) in murine adult hearts. Considering studies demonstrating layer-specific origins of different CPCs, these findings may shed light on possible pathways to study and utilize the diversity of endogenous progenitor cells in the adult heart (5).
OACs vs. LAA exclusion
Medical treatment for stroke prevention in patients with non-valvular AF is governed by risk stratification and balancing the benefit of stroke prevention versus the risk of bleeding complications. Numerous validated risk models have been developed for predicting stroke and bleeding, most common of which is the CHADS2 score for predicting the risk of stroke in non-valvular AF (1,7,8). This integer-based scoring system allots one point for congestive heart failure, diabetes, hypertension, or age 75 years or older, and two points for previous stroke, transient ischemic attack (TIA), or systemic embolic event. The rate of ischemic stroke off warfarin ranges from 0.5% per year in patients with a CHADS2 score of 0; to 7% per year with a CHADS2 score of 5 or 6 (1,8).
According to the guidelines of the American College of Chest Physicians OAC is the optimal choice of antithrombotic therapy for patients with AF at high risk of stroke (CHADS2 score of ≥2). At lower levels of stroke risk, antithrombotic treatment decisions will require a more individualized approach.
In particular, patients with non-rheumatic AF, including those with paroxysmal AF, who are (I) at low risk of stroke [e.g., CHADS2 (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or TIA) score of 0], the suggestion is in favor of no therapy rather than antithrombotic therapy, and for patients choosing antithrombotic therapy, they suggest aspirin rather than OAC or combination therapy with aspirin and clopidogrel; (II) at intermediate risk of stroke (e.g., CHADS2 score of 1), there is a recommendation of OAC rather than no therapy, and suggest OAC rather than aspirin or combination therapy with aspirin and clopidogrel; and (III) at high risk of stroke (e.g., CHADS2 score of ≥2), they recommend OAC rather than no therapy, aspirin, or combination therapy with aspirin and clopidogrel (9). Therapy with OAC, such as warfarin and recently, newer agents such as dabigatran, which is a direct thrombin inhibitor, and rivaroxaban, a factor Xa inhibitor, remains the mainstay of therapy in treating these patients. Nonetheless, OAC does not come without significant risk. Recipients of OAC with either warfarin or any of the newer OAC agents remain at risk for hemorrhagic stroke and life-threatening bleeding (i.e., gastrointestinal, etc.). In addition, those treated with warfarin generally require close outpatient monitoring as well as significant life-style modifications, largely due to this drug’s narrow therapeutic window, requirement for dietary restrictions, and potential for drug-drug interactions (9,10). The above therapeutic limitations along with the severity of AF-related systemic embolic events have led to a great deal of interest in developing novel therapeutic strategies and the concept of LAA exclusion as a means of reducing stroke and other embolic complications in patients with AF. Three general approaches have been devised for excluding the LAA: (I) a surgical approach directed at amputation or ligation of the LAA; (II) a percutaneous endovascular strategy that allows deployment of a device inside the LAA to occlude this structure, and more recently; (III) a percutaneous epicardial ligation technique aimed at externally excluding the LAA (10).
Surgical approach for LAA occlusion
Surgical ligation or amputation of the LAA, which was first performed by Madden in 1949, is now the standard of care in patients undergoing mitral valve surgery or as an adjunct to the maze procedure (1,10). Nowadays the surgical techniques described for LAA occlusion are simple neck ligation, purse string techniques, surgical staplers, and endocardial suturing. Given the adjacent vital anatomic structures and its thin, fragile, delicate wall, amputation of the LAA can be complicated by bleeding or myocardial ischemia. Therefore, reinforcement with autologous pericardium makes amputation a safer means of LAA exclusion.
Another effective method for LAA occlusion described by Hernandez-Estefania et al. is invagination of the LAA and ligation with a purse-string suture and a second, running suture (11). After LAA invagination, the base of the appendage is slightly tied by the purse-string suture delineating the LAA rim and it is then sealed with a running suture along the long axis of the LAA. This method can also be performed during minimally invasive surgery (4). Jayakar et al. described a method using the LigaSureTM Vessel Sealing System (LVSS; Tyco Healthcare, Valleylab, Boulder, CO, USA) which can be applied during both on-, and off-pump procedures. The LVSS uses radiofrequency energy to create tissue fusion of the LAA lumen. After the histological amalgamation, the appendage is ready to be excised and a distal running suture is used as back-up (12). Finally, the AtriClip (Atricure, Inc, Westchester, OH, USA) device is a novel LAA exclusion clip which can safely and atraumatically exclude the LAA during open cardiac surgery (4,13,14).
In addition to the open surgical approach, thoracoscopic LAA ligation using an endoloop snare or stapling has also been described. In the only reported experience, the procedure was successful in 14 of the 15 patients. However, at 42 months, there were two subsequent strokes, corresponding to a stroke rate of 4% per year (1,4,10,13-16).
Percutaneous approach for LAA occlusion
The percutaneous (or else transcatheter) approach has recently been introduced for LAA obliteration. To date, there have been three devices specifically designed for LAA occlusion using an endocardial approach. These include the Percutaneous LAA Transcatheter Occlusion (PLAATO, EV3, Plymouth, MN, USA), the Amplatzer Cardiac Plug (AGA Medical, Plymouth, MN, USA), and the WATCHMAN LAA system (Atritech Inc., Plymouth, MN, USA). But none of the developed LAA closure devices have received FDA approval as of yet (4,10).
Each system has unique features but the implant method is similar for all three. A specific delivery system has been devised for each device, which allows for collapse, repositioning, or removal of the device in the event of suboptimal results.
After venous and trans-septal puncture for percutaneous access to the LA, the implant is advanced under transesophageal echocardiography (TEE) and/or intracardiac echocardiography, fluoroscopic or angiographic guidance through a specially designed sheath and is deployed into the LAA to cover its ostium. The angulation, length, and number of lobes of the LAA as well as the size of its ostium can be assessed through angiography of the LAA. A 20% to 40% oversizing is suggested by some authors to avoid possible implant migration. The “tug” test, consisting of traction forces on the implant, proves its stability within the LAA (4,10).
PLATOO device
The percutaneous left atrial appendage transcatheter occlusion (PLAATO; Appriva Medical, Inc., Sunnyvale, CA, USA) device was the first of the endocardial devices developed specifically for LAA occlusion (17). The PLAATO device held in position by small anchors, disposes a self-expanding nitinol cage covered with a non-thrombogenic polytetrafluoroethylene (ePTFE) membrane to occlude blood flow into the orifice while allowing endothelialization of the device in two to three months. The device diameters range between 15 to 32 mm and were normally selected 20% to 40% larger than the diameter of the LAA ostium (1,3,4,10,17).
A subsequent non-randomized multicenter study, including 111 patients with AF who had contraindications to OAC therapy, demonstrated successful LAA occlusion in 108 patients (97%). Altogether, nine serious adverse events occurred; none were device-related. At six months, successful LAA occlusion was demonstrated in all the patients by TEE, without thromboembolic complications or device embolization or migration. The annual stroke rate was 2.2%, consistent with a 65% relative risk (RR) reduction based on the CHADS2 risk score. More recently, the long-term efficacy of the PLAATO was described by Ussia et al., who reported no embolic events at a mean follow up of 40±10 months Park et al. further verified the stroke risk reduction two years after PLAATO implantation in 71 patients with a mean CHADS2 score of 2.5±1.4 (1,3,4,10,17). In their cohort, they reported no incidence of stroke, whereas seven cases of strokes would have been statistically expected in the absence of OAC. Block et al. reported a 3.8% annual stroke/TIA rate after a 5-year follow-up period when the anticipated CHADS2 annual stroke/TIA rate was estimated at 6.6% (1,3,4,10,17).
However, even though the preliminary experience with this device was promising, the device has been withdrawn from the market due to commercial reasons since 2006 (4,10).
WATCHMANN device
The WATCHMAN LAA closure device (Atritech, Inc., Plymouth, MN, USA) is composed of a self-expanding nitinol frame with a row of fixation barbs around the midperimeter and a 160-mm thick polyethylene membrane being permeable only at the side of the LA (18). Is available in five different sizes with a diameter ranging from 21 to 33 mm? The device size is chosen to be 10% to 20% larger than the LAA diameter to ensure stable device positioning (1,3,4,10,18).
The PROTECT-AF trial was a prospective multicenter randomized trial that compared the WATCHMAN LAA device to OAC therapy with warfarin (control arm) (19). In this study, 707 patients with non-valvular AF from 59 centers in US and Europe were randomized in a device-to-control ratio of 2:1. Patients who received the WATCHMAN device were also treated with warfarin, but only for 45 days post-implant, to allow appropriate device endothelialization. Warfarin was discontinued thereafter. Patients underwent TEE at 45 days, six months, and one year, with follow-up to five years. The trial showed that in patients with AF, LAA closure by the WATCHMAN device was associated with a significant reduction in hemorrhagic stroke risk as compared to warfarin. In addition, all-cause stroke and all-cause mortality outcomes were non-inferior to warfarin. After 1,065 patient-years of follow-up, the rate of these events was 38% lower in the WATCHMAN than the warfarin arm [RR: 0.62, 95% confidence interval (CI): 0.35-1.25]. In particular, the risk of hemorrhagic stroke was significantly lower in the device versus the control arm (RR: 0.09, 95% CI: 0.00-0.45) (1,3,4,10,18,19).
However, implantation of the WATCHMAN device carries a substantial acute procedural risk (18). Among 449 attempted WATCHMAN implants, 12.3% had serious procedural complications, including pericardial effusion requiring drainage or surgery in 5% and acute ischemic stroke due to air or thromboembolism in 1% of the patients. Four patients required device removal due to device embolization or post-implant sepsis. Meanwhile, 86% of patients who received the WATCHMAN were able to discontinue warfarin at day 45. Reasons for continuing warfarin included observation of flow into the LAA or physician discretion. At 12 months, 93% of the recipients of the WATCHMAN device were permanently off warfarin.
A recent study demonstrated that residual flow into the LAA following percutaneous closure with the Watchman device may be common, reported in as many as a third of the recipients of this device at one year. Interestingly, this residual peri-device flow was not shown to be associated with an increased risk of thromboembolism in this study (1,4,10,18).
Amplatzer device
The Amplatzer septal occluder (AGA Medical Corp., Plymouth, MN, USA), is the only device used in LAA exclusion that was not specifically designed for this purpose. This device was initially developed for atrial septal defect closure. However it has been used for LAA occlusion (1,3,4,10,20).
The Amplatzer system was not evaluated further for LAA closure, since a new system (the Amplatzer Cardiac Plug 1) specifically designed for occlusion of the LAA, was subsequently introduced. The device is constructed from a nitinol mesh and Dacron, and consists of a lobe and a disk connected by a central waist. There are 12 stabilizing wires equally spaced about the main disc. The sizes of the lobes range between 16 to 30 mm. The lobe is designed to conform to the inner wall of the LAA with a depth of 10 mm or more, and provides secure device placement and retention by the stabilizing wires. The size of the device should be at least 2 mm larger than the LAA landing zone diameter. The device is retrievable and can be redeployed. Successful device deployment is generally confirmed by TEE. This device received a CE mark in 2008, but is not currently approved in the US (20).
In order to facilitate the implantation process and minimize the occurrence of complications a new generation of the Amplatzer™ Cardiac Plug, Amulet™ (ACP 2) has been designed, without changing the main design of the ACP 1. The first-in-human percutaneous LAA closure using the ACP 2 was performed at the Montreal Heart Institute on July 19 2012 (21).
The ACP 2 is a self-expanding device specifically designed for LAA closure. The main design, made of a nitinol mesh with two polyester patches sewn on to a distal lobe and a proximal disc connected by a short waist, has been carried over from the first generation device. Similarly to the first generation, the ACP 2 is implanted through the femoral vein via the trans-septal technique, and is fully retrievable and repositionable (21). The modifications leading to the ACP 2 design are the following:
- No need to prepare and load the device as it comes pre-loaded inside the delivery system;
- The length of the distal lobe is 2 to 3 mm longer than the ACP 1;
- The stabilizing wires (hooks) are stiffer;
- The number of stabilizing wires has been increased from six pairs in the ACP 1 to up to 10 pairs;
- The diameter of the proximal disc has been increased in the ACP 2, now being 6 to 7 mm greater than the distal lobe diameter compared to 4 to 6 mm in the ACP 1;
- The waist between the distal lobe and the proximal disc has also been lengthened from 4 mm in the ACP 1 to 5.5 or 8 mm depending on the size of the device;
- The attaching screw on the proximal disc has been inverted;
- The ACP 2 has a new delivery cable;
- Larger sizes are available (31 and 34 mm).
These modifications to the design of the original device were made to facilitate implantation and improve sealing performance. There is of course need of studies in order to prove its efficacy and safety (20,21).
Percutaneous epicardial occlusion—Lariat device
Recently, the Lariat suture delivery device (SentreHEART, Inc., Palo Alto, CA, USA) by Bartus et al. has been introduced with promising results, which is a closed-chest, percutaneous, epicardial catheter-based LAA ligation technique (22). This device consists of a pre-tied suture contained on a closure snare which is guided via a catheter epicardially over the LAA. This technique involves, a combined endocardial and percutaneous subxiphoid epicardial magnet-tipped wire-guided approach, which are advanced under fluoroscopic guidance, to ‘snare’ and ligate the LAA at its ostium and a catheter positioned trans-septal. A balloon is placed at the ostium of the LAA to mark it. Then, the Lariat device is guided through the catheter over the LAA in order to close the LAA by suture ligation (4,10,22). The position of the snare at the LAA ostium is guided by the balloon catheter positioned inside the ostium of the LAA and confirmed by TEE. Following placement verification, the snare is closed and tightened using the suture tightener. A repeat left arteriogram is performed to ensure complete LAA closure (22).
In the initial report, 10 of the 11 patients successfully underwent acute LAA ligation using this novel approach (nine with percutaneous epicardial access and two with simultaneous open surgical MV replacement) (22).
A potential advantage of this strategy is the lack of need for OAC immediately following the procedure. Unlike intracardiac implants that require anticoagulation to protect against thrombus formation while endothelialization occurs, the LARIAT snare device can offer immediate LAA closure without implantation of a foreign object inside the LA. In addition, it may also overcome the potential complications related to implantable devices such as cardiac perforation, erosion, and device migration and embolization. On the other hand, the main procedural limitation of this approach is the requirement to obtain epicardial access in all patients—a technique that is not familiar to many operators, and often not possible in patients with a prior history of cardiac surgery and those with pericardial adhesions. There are also anatomical considerations, such as large LAA, posteriorly rotation of LAA, superiorly orientated LAA lobes and posteriorly rotated hearts, with respect to successful ligation of the LAA using the LARIAT snare device (1,4,10,22).
Limitations
One limitation to LAA closure is that it may be incomplete. Several TEE studies reported incomplete LAA closure rates of 10% to 80% (10). The highest success of complete LAA closure can be achieved with surgical excision, and the lowest with surgical exclusion (suture or staple ligation). More recently, the randomized controlled pilot Left Atrial Appendage Occlusion Study (LAAOS), assessed the safety and efficacy of LAA ligation during coronary artery bypass surgery (10). The study demonstrated that complete LAA closure was quite challenging and operator dependent. Postoperative TEE revealed complete occlusion of 45% with sutures and of 72% with staples. Failure to achieve complete occlusion was secondary to the presence of a residual stump and not due to leaks. Incomplete LAA ligation may in turn result in future thrombus formation in up to 50%, and lead to clinically relevant thromboembolic sequelae in as many as 8% of patients. Patients with unsuccessful or incomplete LAA closure continue to be exposed to the risk of thromboembolic events originating from the LAA (1,4,10).
According to results of transesophageal echocardiograph, incomplete LAA occlusion does not appear to be secondary to an enlarged LA or significant mitral valve regurgitation (4). Furthermore, neither the appendage size nor the surgical procedure and operative approach predispose to an incompletely ligated LAA. Several surgical factors may be responsible for incomplete LAA ligation. First, the empty and unstretched appendage during cardiopulmonary bypass may conceal the fact that the running sutures used may not start and end exactly at the most distal edges of the LAA leading to incomplete ligation. Moreover, although deep suture bites must be avoided not to inadvertently injure the circumflex coronary artery or its branches, shallower suture bites may dehisce when the LA is once again filled and stretched. A smooth endocardial surface which inhibits scar formation, the continuous secretion of the LAA endocardium resulting in leakage, the OAC-related incomplete closure of the sutured orifice and improper surgical suture techniques are additional reasons why incomplete occlusion may occur (4,10).
Other limitations of LAA ligation are the potential complications. Atrial tears and related bleeding are important potential risks complicating surgical LAA occlusion. Studies reported that when the atrial appendage is removed, the LA becomes less compliant resulting in significant changes in left ventricular and left atrial filling and in atrial function (5,23). Thirty percent of total cardiac ANF derives from atrial appendages. Hence, another “functional” complication of the LAA resection is the impairment of cardiac function due to ANF excretion reduction characterized by heart failure and fluid retention (23). In fact, bilateral atrial appendectomy has led to decreased atrial natriuretic peptide release and subsequent fluid retention which is attenuated when the right atrial appendage remains untouched (4,10,23).
The percutaneous approach is also associated with several procedural or device-related adverse events such as major bleeding, pericardial effusion due to cardiac perforation, and device embolisation. Over- or under-sizing, device migration, and dislodgement constitute additional potential complications. Air embolism and vascular access injuries may also occur. Iatrogenic small atrial septal defects usually disappear within six months after implantation (1,3,4,10).
When LAA must be excluded?
Currently guidelines (ACC/AHA, ESC, CCS, EACTS) regarding LAA exclusion are scarce. Although occlusion of LAA appears to be as a promising approach for stroke prevention in AF, easily performed during open heart surgery, its efficacy and its safety are controversial (4,13,24). Current evidence does not clearly favor LAA occlusion which is potentially harmful especially in case of incomplete exclusion (13,24). Moreover, emboli in AF do not exclusively originate from the LAA. There are multiple additional sources of emboli in AF patients due to atherosclerosis, such as the aorta, left ventricle, and cerebral vasculature. However, more than 90% of all left atrial thrombi in patients with non-rheumatic AF have been shown to derive from LAA (25). Current results report that the LAA occlusion is associated with reduced long-term stroke rates and with decreased recurrence in patients experiencing stroke despite being under OAC (24).
If complete LAA occlusion can safely take place in patients experiencing AF, it should be performed (26). The American College of Cardiology/American Heart Association guidelines for the management of patients with valvular heart disease include LAA obliteration/exclusion when mitral valve surgery is performed (27). ACC/AHA/ESC guidelines for the management of patients with AF also include LAA exclusion from systemic circulation whenever possible during cardiac surgery in patients at risk of developing postoperative AF (28). LAA is also occluded/excluded when Maze procedures for ablation of AF are performed (26,29). Finally, LAA exclusion is also indicated in patients with chronic AF who have a contraindication to chronic anticoagulation (16,28,30). However, ESC/EACTS guidelines on the management of valvular heart disease of 2012, indicates that, no evidence supports the systematic surgical closure of the LAA, unless as part of AF ablation surgery (31-34).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Singh IM, Holmes D. Left atrial appendage closure. Curr Cardiol Rep 2010;12:413-21. [PubMed]
- Leal S, Moreno R, de Sousa Almeida M, et al. Evidence-based percutaneous closure of the left atrial appendage in patients with atrial fibrillation. Curr Cardiol Rev 2012;8:37-42. [PubMed]
- Cruz-Gonzalez I, Yan BP, Lam YY. Left atrial appendage exclusion: state-of-the-art. Catheter Cardiovasc Interv 2010;75:806-13. [PubMed]
- Apostolakis E, Papakonstantinou NA, Baikoussis NG, et al. Surgical strategies and devices for surgical exclusion of the left atrial appendage: a Word of caution. J Card Surg 2013;28:199-206. [PubMed]
- Leinonen JV, Emanuelov AK, Platt Y, et al. Left atrial appendages from adult hearts contain a reservoir of diverse cardiac progenitor cells. PLoS One 2013;8:e59228. [PubMed]
- Al-Saady NM, Obel OA, Camm AJ. Left atrial appendage: structure, function, and role in thromboembolism. Heart 1999;82:547-54. [PubMed]
- Stroke Risk in Atrial Fibrillation Working Group. Comparison of 12 risk stratification schemes to predict stroke in patients with nonvalvular atrial fibrillation. Stroke 2008;39:1901-10. [PubMed]
- Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001;285:2864-70. [PubMed]
- You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e531S-75S.
- Aryana A, Saad EB. d’avila A. Left atrial appendage occlusion and ligation devices: what is available, how to implement them, and how to manage and avoid complications. Curr Treat Options Cardiovasc Med 2012;14:503-19. [PubMed]
- Hernandez-Estefania R, Levy Praschker B, Bastarrika G, et al. Left atrial appendage occlusion by invagination and double suture technique. Eur J Cardiothorac Surg 2012;41:134-6. [PubMed]
- Jayakar D, Gozo F, Gomez E, et al. Use of tissue welding technology to obliterate left atrial appendage--novel use of Ligasure. Interact Cardiovasc Thorac Surg 2005;4:372-3. [PubMed]
- Hanke T, Sievers HH, Doll N, et al. Surgical closure of the left atrial appendage in patients with atrial fibrillation. Indications, techniques and results. Herzschrittmacherther Elektrophysiol 2013;24:53-7. [PubMed]
- Starck CT, Steffel J, Emmert MY, et al. Epicardial left atrial appendage clip occlusion also provides the electrical isolation of the left atrial appendage. Interact Cardiovasc Thorac Surg 2012;15:416-8. [PubMed]
- Ohtsuka T, Ninomiya M, Nonaka T, et al. Thoracoscopic stand-alone left atrial appendectomy for thromboembolism prevention in nonvalvular atrial fibrillation. J Am Coll Cardiol 2013;62:103-7. [PubMed]
- Blackshear JL, Johnson WD, Odell JA, et al. Thoracoscopic extracardiac obliteration of the left atrial appendage for stroke risk reduction in atrial fibrillation. J Am Coll Cardiol 2003;42:1249-52. [PubMed]
- Sievert H, Lesh MD, Trepels T, et al. Percutaneous left atrial appendage transcatheter occlusion to prevent stroke in high-risk patients with atrial fibrillation: early clinical experience. Circulation 2002;105:1887-9. [PubMed]
- Möbius-Winkler S, Sandri M, Mangner N, et al. The WATCHMAN left atrial appendage closure device for atrial fibrillation. J Vis Exp 2012;3671. [PubMed]
- Fountain RB, Holmes DR, Chandrasekaran K, et al. The PROTECT AF (WATCHMAN left atrial appendage system for embolic PROTECTion in patients with atrial fibrillation) trial. Am Heart J 2006;151:956-61. [PubMed]
- Nietlispach F, Gloekler S, Krause R, et al. Amplatzer left atrial appendage occlusion: single center 10-year experience. Catheter Cardiovasc Interv 2013;82:283-9. [PubMed]
- Freixa X, Chan JL, Tzikas A, et al. The amplatzer™ cardiac plug 2 for left atrial appendage occlusion: novel features and first-in-man experience. EuroIntervention 2013;8:1094-8. [PubMed]
- Bartus K, Han FT, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013;62:108-18. [PubMed]
- Hara H, Virmani R, Holmes DR Jr, et al. Is the left atrial appendage more than a simple appendage? Catheter Cardiovasc Interv 2009;74:234-42. [PubMed]
- Dawson AG, Asopa S, Dunning J. Should patients undergoing cardiac surgery with atrial fibrillation have left atrial appendage exclusion? Interact Cardiovasc Thorac Surg 2010;10:306-11. [PubMed]
- Onalan O, Crystal E. Left atrial appendage exclusion for stroke prevention in patients with nonrheumatic atrial fibrillation. Stroke 2007;38:624-30. [PubMed]
- Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation developed in partnership with the European Heart Rhythm Association (EHRA) and the European Cardiac Arrhythmia Society (ECAS); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Endorsed and approved by the governing bodies of the American College of Cardiology, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, and the Heart Rhythm Society. Europace 2007;9:335-79. [PubMed]
- American College of Cardiology, American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease), Society of Cardiovascular Anesthesiologists, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006;48:e1-148. [PubMed]
- Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: full text: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace 2006;8:651-745. [PubMed]
- Holmes DR Jr, Schwartz RS. Left atrial appendage occlusion eliminates the need for warfarin. Circulation 2009;120:1919-26; discussion 1926.
- European Heart Rhythm Association, European Association for Cardio-Thoracic Surgery, Camm AJ, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace 2010;12:1360-420. [PubMed]
- Vahanian A, Iung B. The new ESC/EACTS guidelines on the management of valvular heart disease. Arch Cardiovasc Dis 2012;105:465-7. [PubMed]
- Taylor J. ESC/EACTS guidelines on the management of valvular heart disease. Eur Heart J 2012;33:2371-2. [PubMed]
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012;42:S1-44. [PubMed]
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC), European Association for Cardio-Thoracic Surgery (EACTS), Vahanian A, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451-96. [PubMed]


