Nonintubated thoracoscopic surgery using regional anesthesia and vagal block and targeted sedation
IntroductionOther Section
Since the introduction of video-assisted thoracoscopic surgery (VATS), it has become a preferred alternative to treat patients with thoracic diseases (1,2). For decades, intubated general anesthesia with one-lung ventilation has been considered mandatory during VATS (3,4). However, complications and adverse effects following tracheal intubation and one-lung ventilation are inevitable, including intubation-related airway trauma, ventilation-induced lung injury, residual neuromuscular blockade, impaired cardiac performance, and postoperative nausea and vomiting (5-10).
To reduce the adverse effects of tracheal intubation and general anesthesia, thoracoscopic surgery without tracheal intubation has been recently employed for management of pneumothorax (11), resection of pulmonary nodules (12-14), resection of solitary metastases (15), lung volume reduction surgery (16), lobectomy, and segmentectomy (17-20). The results achieved for these early surgeries are encouraging.
Although the feasibility of thoracoscopic surgery via nonintubated anesthesia was demonstrated in some reports, most of them are limited to small number of cases. In this study, we reported our experience of 446 consecutive patients undergoing nonintubated VATS in a 4-year period of time to evaluate the feasibility, safety, and indication of this innovative technique in a tertiary medical center in Taiwan. The anesthesia and surgical techniques were also described.
Patients and methodsOther Section
Study design and patients
The medical records of all patients who underwent nonintubated VATS at National Taiwan University Hospital from August 2009 to August 2013 were retrospectively reviewed. The thoracic surgical team, both surgeons and anesthesiologists, selected the cases upon review of the medical records. Patients considered appropriate for non-intubated thoracoscopic surgery met the same criteria as for intubated single-lung ventilation, including patients with clinical stage I or II non-small-cell lung cancer, metastatic lung cancer, or benign lung tumor. The tumors were peripherally located and smaller than 6 cm in diameter, without evidence of chest wall, diaphragm, or main bronchus involvement. Patients with primary or secondary spontaneous pneumothorax were also suitable for nonintubated thoracoscopic surgery. Patients with American Society of Anesthesiologists (ASA) scores of greater than three, bleeding disorders, sleep apnea, or unfavorable airway or spinal anatomy were contraindicated for nonintubated VATS in our hospital. Patient consent was obtained after explaining the type of anesthesia and the surgical procedure.
The operation methods used had included conventional VATS or needlescopic VATS. All patients were managed by a single thoracic surgical team using the same clinical protocols, care patterns, and perioperative orders.
Anesthetic setting, induction, and maintenance
Anesthetic techniques were described previously (13,14,17-20). Briefly, all patients were pre-medicated with fentanyl 50-100 µg intravenously. Standard monitoring included electrocardiogram, arterial blood pressure, pulse oximetry, and respiratory rate. The end-tidal carbon dioxide was measured by insertion of a detector into one nostril. A bispectral index sensor (BIS Quatro, Aspect Medical System, Norwood, MA, USA) was applied to the forehead of each patient to monitor the level of consciousness (21). The patients were then sedated with intravenous propofol (Fresfol 1%, Fresenius Kabi GmbH, Graz, Austria) using a target-controlled infusion method (Injectomat® TIVA Agilia, Fresenius Kabi GmbH, Graz, Austria). The level of sedation was set to achieve a bispectral index value between 40 and 60 (21), and incremental intravenous injections of fentanyl 25 µg were given to maintain a respiratory rate between 12 to 20 breaths/min. The patients were placed in the lateral decubitus position. During the procedure, patients spontaneously breathed oxygen through a ventilation mask.
Regional anesthesia was achieved by thoracic epidural anesthesia between 2009 and 2012. From March 2012, we began to use internal intercostal blockade as an alternative for thoracic epidural anesthesia because it is easier and time saving. Thoracic epidural anesthesia was performed by insertion of an epidural catheter at the T5/6 thoracic interspace to achieve a sensory block between the T2 and T9 dermatomes before sedation, and was maintained by continuous infusion of 2% lidocaine. Thoracoscopic intercostal nerve block was produced by infiltration of 0.5% bupivacaine (1.5 mL for each intercostal space) from the third to the eighth intercostal nerve under the parietal pleura, 2 cm laterally to the sympathetic chain, with a 25-G top-winged infusion needle.
During the procedure, patients breathed O2 through a ventilation mask, keeping oxygen saturation above 90%. An iatrogenic pneumothorax was made by creating incisions through the chest wall for thoracoscopy and the ipsilateral lung collapsed gradually. To inhibit coughing during thoracoscopic manipulation in selected patients, intrathoracic vagal blockade was produced by infiltration of 3 mL of 0.5% bupivacaine adjacent to the vagus nerve at the level of the lower trachea for right-sided operations and at the level of the aortopulmonary window for left-sided operations, under direct thoracoscopic vision. This procedure effectively inhibited the cough reflex for three or more hours and was mandatory for lobectomy and segmentectomy, especially before anatomical dissection of the pulmonary hilum. Repeated bupivacaine infiltration was occasionally needed in prolonged operations.
During wound closure and chest tube insertion, propofol infusion was stopped. After the patient was fully awake, the patient was asked to breathe deeply and cough to re-expand the collapsed lung.
Technique of thoracoscopic surgery
Thoracoscopic lobectomy, segmentectomy, or wedge resection was performed using a 3-port method, as described by McKenna (22). In brief, the patient was positioned in the full-lateral decubitus position, with slight flexion of the table at the level of the mid-chest. The thoracoscope was placed into the seventh or eighth intercostal space in the midaxillary line. A working port was placed in the sixth or seventh intercostal space in an auscultatory triangle, and an anterior 3 cm incision was placed anteriorly in the fifth intercostal space. After collapse of the lung, incomplete fissures, pulmonary vessels, and bronchi were divided with endoscopic stapling devices. The resected specimen was removed in an organ retrieval bag through the utility incision. After staging mediastinal lymph node dissection, a 28-French chest tube was placed through the lowest incision. Rib spreading, rib cutting, and retractor use were avoided in all patients, except when conversion to thoracotomy was required.
Technique of needlescopic VATS
Needlescopic VATS was mainly used for biopsy of undiagnosed peripheral lung nodules. The technique was described previously (13). An incision of about 15 mm in length was made in the sixth intercostal space on the midaxillary line and a 12-mm thoracic port was inserted through the incision. Two or three small skin punctures were made and mini-ports were inserted for the needlescopic instruments (3-mm instruments, Olympus, Tokyo, Japan). Initially, the 10-mm telescope and two mini-endograspers were used to identify the nodule. Once the nodule was identified, it was stabilized using the mini-endograsper. The mini-endograsper in the other mini-port was withdrawn and a needlescope was introduced to visualize the tumor. The 10-mm telescope was then withdrawn and a 45-mm endoscopic stapler was introduced for partial lung resection including the nodule. Resected tissue was placed into a bag inserted through the 12-mm port and was taken out of the thoracic cavity. Upon completion of the procedure, a chest tube was inserted via the 12-mm port.
Anesthetic conversion
The attending surgeon and anesthesiologist decided whether or not to convert nonintubated anesthesia to intubated general anesthesia with one lung ventilation in cases of ineffective analgesia, profound respiratory movement, massive pleural adhesions, persistent hypoxemia (SPO2 <80%), unstable hemodynamic status, or intraoperative bleeding requiring thoracotomy. When conversion was indicated, the surgical wounds were sealed with transparent waterproof dressings (Tegaderm Film, 3M Health Care, Neuss, Germany) after insertion of a chest tube to re-expand the lung. A single-lumen endotracheal tube was inserted under the guidance of a bronchoscope, followed by insertion of a bronchial blocker without changing the patient’s position.
Postoperative analgesics and care
Postoperative analgesics were administered by patient controlled epidural or intravenous infusion of analgesics. Chest radiography was performed immediate or the next morning. Drinking and meal intake were resumed 2-4 hours after surgery. The chest tube was removed if no air leak was present and drainage was less 200 mL in a 24-hour period.
Data collection and analyses
The data including patient demographics, complications, and the surgical results were collected from the institutional database, anesthesia and surgical notes, and the medical and nursing records.
ResultsOther Section
From August 2009 through August 2013, nonintubated VATS was performed on 446 patients. Among them, 156 patients underwent thoracoscopic intercostal nerve block, vagal block, and targeted sedation for management of their pulmonary diseases. The remaining 290 patients underwent thoracic epidural anesthesia, vagal block, and sedation for nonintubated VATS. The demographic data are reported in Table 1. The mean patient age was 56.9 years and 181 patients (40.6%) were male. Four patients received bilateral VATS for lesions in both lungs. Needlescopic VATS was performed in 57 patients (12.8%) for resection of peripheral lung nodules while the remaining patients underwent conventional VATS. The median anesthetic induction time was 30 minutes (range, 15 to 60 minutes) by thoracic epidural anesthesia and was 10 minutes (range, 5 to 30 minutes) by internal intercostal blockade. The operation procedures have included lobectomy in 189 patients (42.4%), wedge resection in 229 patients, and segmentectomy in 28 cases. Most of the patients were diagnosed as non-small cell lung cancer (59.0%).
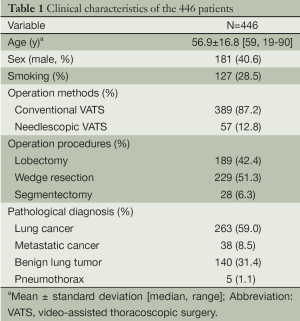
Full table
The operative and anesthetic results are shown in Table 2. After the surgery, anesthetic side effects were noted in 28 patients (6.3%), including vomiting, sore throat, and headache. Operation complications were noted in 14 patients (3.1%), including air leaks >5 days, bleeding, and pneumonia. Sixteen patients (3.6%) required conversion to tracheal intubation because of significant mediastinal movement (seven patients), persistent hypoxemia (two patients), dense pleural adhesions (two patients), ineffective epidural anesthesia (two patients), bleeding (two patients), and tachypnea (one patient) (Table 3). Conversion to a thoracotomy was required in one patient with blood transfusion due to bleeding during dissection of pulmonary artery. No mortality was noted in this study.
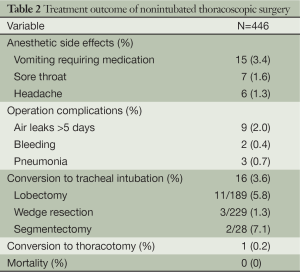
Full table

Full table
DiscussionOther Section
Recent reports and our previous studies have suggested that many surgical thoracic procedures are feasible using nonintubated anesthetic techniques, with patients under awake or sedative status (11-20). Our experience of a lot of number of cases with satisfactory results and low conversion rates also showed that nonintubated thoracoscopic lobectomy, segmentectomy, and wedge resection are safe and can be effective alternatives to intubated thoracoscopic procedures.
Concerns might arise with the use of nonintubated anesthesia for pulmonary resection, especially for complicated procedures entailing fine vascular dissection such as lobectomy or segmentectomy. First, prolonged one-lung spontaneous breathing during surgery could lead to hypoxia and hypercapnia. Secondly, cough reflex and unexpected lung movement can be encountered during pulmonary hilar manipulation. Thirdly, conversion to general anesthesia with intubation could be required occasionally (17).
To our surprise, SPO2 was maintained at 95% or more during the whole operation in most cases. Hypercapnea was noted in some patients, especially when the surgery was long. Our experience showed that hypercapnia was permissive and did not affect the hemodynamics and surgical procedures, which was comparable to a recently published report by Dr. Dong et al. (23).
Cough reflex and unexpected lung movement during manipulation of the pulmonary hilum was hazardous when performing nonintubated lobectomy or segmentectomy. Using intrathoracic vagal blockade, the cough reflex and unexpected lung movement could be effectively abolished, without affecting the heart rate and blood pressure (17).
Although nonintubated thoracoscopic procedures could provide an attractive alternative in managing lung diseases, sixteen patients (3.6%) required conversion to intubated one-lung ventilation because of significant mediastinal movement, persistent hypoxemia, dense pleural adhesions, ineffective epidural anesthesia, bleeding, and tachypnea. Our results suggested that obese patients with body mass index >30 are at a high risk of anesthetic conversion because it usually associated with vigorous spontaneous breathing and significant mediastinal movement. We suggest that proper patient selection, accumulated experience by performing minor non-intubated thoracoscopic procedures, and conversion to intubated general anesthesia without hesitation are mandatory to decrease the risk of emergency intubation and complications, especially at the beginning of the learning curve.
In our cohort, almost two third of the patients were women. We believe that nonintubated thoracoscopic surgery is most applicable in small body-sized female patients. These patients are prone to have small tracheal caliber and are susceptible to intubation-related complications such as sore throat, hoarseness, and subglottic stenosis, especially when double-lumen endotracheal tubes are used. Using the nonintubated technique, we found that the rates of postoperative sore throat were significantly decreased. It is reasonable to suggest that the incidence of hoarseness and tracheal injury could also have been lower, although they were not investigated in this study.
When performing simple thoracoscopic procedures with short operation duration such as wedge resection, previous studies showed awake surgery is feasible and safe (11,12). We did not use awake technique because when the procedures become prolonged or complicated owing to unexpected reasons, conversion to intubated anesthesia with one-lung ventilation is required. Using our nonintubated technique with targeted sedation, major and complicated thoracoscopic procedures such as lobectomy and segmentectomy can be performed without conversion.
Thanks to the avoidance of tracheal intubation and muscle relaxants, the anesthetic side effects were minimal in our patients. Most of our patients resume oral intake and ambulation around two hours after the operation. The rates of postoperative sore throat and vomiting are also lower compared with intubated patients in our previous studies (17,18).
We acknowledge that this study was limited by its retrospective design and the lack of a control group for comparison. Further detailed investigations by prospective controlled designs are needed to elucidate the impact of the different anesthesia protocols on perioperative outcomes, cancer metastasis status, and overall survival.
ConclusionsOther Section
Our results have suggested that nonintubated thoracoscopic surgery is safe and technically feasible. Avoidance of intubation, mechanical ventilation, and muscle relaxants was reflected in less intubation-associated discomfort, and immediate return to many daily life activities including drinking, eating, and walking. Although the long-term benefits remain unclear, we suggest that it can potentially be an attractive alternative of intubated one-lung ventilated thoracoscopic surgery in managing patients with a variety of thoracic diseases, after prospective, randomized data become available.
AcknowledgementsOther Section
Disclosure: The authors declare no conflict of interest.
ReferencesOther Section
- Luo Q, Han Q, Chen X, et al. The diagnosis efficacy and safety of video-assisted thoracoscopy surgery (VATS) in undefined interstitial lung diseases: a retrospective study. J Thorac Dis 2013;5:283-8. [PubMed]
- Jurado J, Javidfar J, Newmark A, et al. Minimally invasive thymectomy and open thymectomy: outcome analysis of 263 patients. Ann Thorac Surg 2012;94:974-81; discussion 981-2. [PubMed]
- Ovassapian A. Conduct of anesthesia. In: Shields TW, LoCicero J, Ponn RB. eds. General thoracic surgery. Philadelphia: Lippincott Williams & Wilkins, 2000:327-44.
- Campos JH. Current techniques for perioperative lung isolation in adults. Anesthesiology 2002;97:1295-301. [PubMed]
- Murphy GS, Szokol JW, Avram MJ, et al. Postoperative residual neuromuscular blockade is associated with impaired clinical recovery. Anesth Analg 2013;117:133-41. [PubMed]
- Murphy GS, Szokol JW, Marymont JH, et al. Residual neuromuscular blockade and critical respiratory events in the postanesthesia care unit. Anesth Analg 2008;107:130-7. [PubMed]
- Gothard J. Lung injury after thoracic surgery and one-lung ventilation. Curr Opin Anaesthesiol 2006;19:5-10. [PubMed]
- Fitzmaurice BG, Brodsky JB. Airway rupture from double-lumen tubes. J Cardiothorac Vasc Anesth 1999;13:322-9. [PubMed]
- Campos JH, Hallam EA, Van Natta T, et al. Devices for lung isolation used by anesthesiologists with limited thoracic experience: comparison of double-lumen endotracheal tube, Univent torque control blocker, and Arndt wire-guided endobronchial blocker. Anesthesiology 2006;104:261-6, discussion 5A.
- Ishikawa S, Lohser J. One-lung ventilation and arterial oxygenation. Curr Opin Anaesthesiol 2011;24:24-31. [PubMed]
- Pompeo E, Tacconi F, Mineo D, et al. The role of awake video-assisted thoracoscopic surgery in spontaneous pneumothorax. J Thorac Cardiovasc Surg 2007;133:786-90. [PubMed]
- Pompeo E, Mineo D, Rogliani P, et al. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg 2004;78:1761-8. [PubMed]
- Tseng YD, Cheng YJ, Hung MH, et al. Nonintubated needlescopic video-assisted thoracic surgery for management of peripheral lung nodules. Ann Thorac Surg 2012;93:1049-54. [PubMed]
- Tsai TM, Chen JS. Nonintubated thoracoscopic surgery for pulmonary lesions in both lungs. J Thorac Cardiovasc Surg 2012;144:e95-7. [PubMed]
- Pompeo E, Mineo TC. Awake pulmonary metastasectomy. J Thorac Cardiovasc Surg 2007;133:960-6. [PubMed]
- Mineo TC, Pompeo E, Mineo D, et al. Awake nonresectional lung volume reduction surgery. Ann Surg 2006;243:131-6. [PubMed]
- Chen JS, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lobectomy for lung cancer. Ann Surg 2011;254:1038-43. [PubMed]
- Wu CY, Chen JS, Lin YS, et al. Feasibility and safety of nonintubated thoracoscopic lobectomy for geriatric lung cancer patients. Ann Thorac Surg 2013;95:405-11. [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lung resection: a 3-year experience with 285 cases in a single institution. J Thorac Dis 2012;4:347-51. [PubMed]
- Hung MH, Hsu HH, Chen KC, et al. Nonintubated thoracoscopic anatomical segmentectomy for lung tumors. Ann Thorac Surg 2013;96:1209-15. [PubMed]
- Chan MT, Cheng BC, Lee TM, et al. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol 2013;25:33-42. [PubMed]
- McKenna RJ Jr. Lobectomy by video-assisted thoracic surgery with mediastinal node sampling for lung cancer. J Thorac Cardiovasc Surg 1994;107:879-81; discussion 881-2. [PubMed]
- Dong Q, Liang L, Li Y, et al. Anesthesia with nontracheal intubation in thoracic surgery. J Thorac Dis 2012;4:126-30. [PubMed]


