Transcatheter closure of atrial septal defect: principles and available devices
Introduction
Atrial septal defect (ASD) is one of the most common congenital heart diseases (CHDs) and accounts for the most common CHD in adults (1). There has been a shift of paradigm for therapeutic strategy of ASD over the last decades. Techniques and devices for transcatheter treatment have been evolved and refined; as a result, device closure of ASD is currently accepted as the treatment of choice in most patients with secundum ASD, showing excellent efficacy as well as lower complication rate comparing to surgery (2,3).
Extensive experiences have verified safety and usefulness of the procedure, and established general principle for device closure of ASD including patient selection, peri-procedural assessment as well as procedural technique with various measures to prevent potential complications. However, unanticipated difficulties and un-negligible risks may be encountered during this “usually straightforward” procedure, and every effort has to be made to promote the efficacy and safety of the procedure on the basis of accurate knowledge for procedural principles, solutions for specific problems as well as characteristic features of available devices.
Principles and issues of device closure of ASD
Patient selection is an important initial step for a successful treatment. Procedure in the catheterization laboratory may be summarized stepwise; (I) hemodynamic study and assessment of morphologic characteristics of the defect; (II) establishment of procedural strategy including procedure-guiding modality and equipment to be used; (III) selection of optimal type and size of device; (IV) device implantation with cautions for potential complications including air embolism, damage to cardiac/vascular structures; (V) post-implantation assessment of the final result. After a successful procedure, appropriate patient education and follow-up are also essential parts of the treatment. Details and special considerations for each step of the procedure have well been described previously (4). There are individual issues which are often in debate including procedure-guiding modalities, sizing the defect, and closing complex defects.
Issues on imaging guidance and defect sizing
Although there have been studies reporting device closure guided only by either fluoroscopy or echocardiography (5-7), it is generally recommended to perform the procedure under both fluoroscopic and echocardiographic guidance. For echocardiographic guidance, transesophageal echocardiography (TEE) has long been the standard modality for ASD closure. However, intracardiac echocardiography (ICE) is gradually replacing the role of TEE recently. Transthoracic echocardiography (TTE) may also be used especially in patients with good windows for echocardiography such as small children (8).
There has been debate on the necessity of balloon sizing for selection of device size. Balloon sizing may be skipped in suitable defects with sufficient surrounding rims (9); however, it has long been regarded as an essential step of the procedure (10). Indeed, balloon sizing may provide more information than averaged size of the defect including compliance of surrounding rims and presence of additional defect.
While the balloon stretched diameter or balloon occlusive diameter were used in balloon sizing in the past, currently stop flow diameter (SFD) is recommended as the standard measurement to avoid oversizing (11). In self-centering devices such as the Amplatzer Septal Occluder (ASO) (St. Jude Medical, St. Paul, MN, USA), the recommended device size is the same or slightly larger (<2 mm) than the SFD. Nevertheless, the selection for device size should be individualized considering deficiency of rims, spatial relationship with nearby cardiac structures and size of the heart. In cases with aortic rim deficiency, the usual recommendation is to avoid an “oversized” device because of the potential risk of erosion (11). On the other hand, in cases with inferior vena cava (IVC) rim deficiency with higher risk of device embolization, use of an “undersized” device should be avoided. In case of using a non-self-centering device such as the Gore Septal Occluder (GSO) (WL Gore & Associates, Inc., Flagstaff, AZ, USA), a device twice the size of the defect is recommended (12), and the GSO is not recommended for defects >18 mm (13).
Complex lesions
Large defect
Large size of the defect may be the most common cause of difficulties in ASD closure using a device. The main problems are prolapse of left atrial (LA) disk of the device into the right atrium (RA) before proper positioning in the septum (14) and difficulty in sizing the defect. Device size is frequently selected by estimation rather than measurement of balloon diameter due to non-visualization of the whole defect in a single echocardiographic plane, difficulty in stabilization of the sizing balloon (melon-seeding or milking) and unavailability of sizing balloon larger than 34 mm.
Various modified implantation techniques have been suggested to overcome the problem with LA disk prolapse (Table 1). An operator should be familiar with his/her own technique to overcome this problem. The balloon-assisted technique may be helpful even in cases when other methods failed.
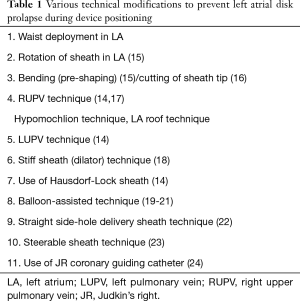
Full table
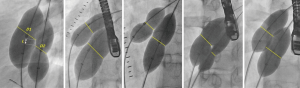
Rim deficiencies (Figure 1A,B,C,D,E)
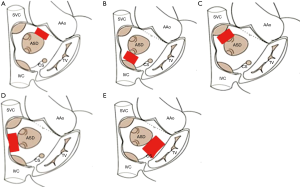
Deficiency in the surrounding rim(s) is frequently associated with large defects, and may potentially increase the risk of complications such as device embolization, erosion and encroachment of device onto nearby cardiac structures.
Aortic rim (antero-superior rim, Figure 1A) deficiency is most common rim deficiency (25) and device implantation is frequently interfered by LA disk prolapse. Erosion risk is higher in aortic rim deficiency as well as device oversizing (11), thus device selection has to be refrained from undue oversizing.
IVC rim (posteroinferior rim, Figure 1B) deficiency is second most common among rim deficiencies and associated with higher risk of device embolization (26). In case with this rim deficiency, under-sizing of the device may further increase the risk of device embolization, and should be avoided. It is difficult to visualize IVC rim with TEE guidance, so ICE is preferable imaging modality in patients with IVC rim deficiency (27); however, so called ‘modified retroflexed view’ may be helpful to visualize IVC rim with TEE guidance (28).
Superior vena cava (SVC) rim (posterosuperior rim, Figure 1C) deficiency is a rare condition and may interfere with device positioning (29). When the rim deficiency is extended from SVC rim to aortic rim, this indicates the defect is located superiorly in the atrial roof and may carry higher risk of erosion (11,30).
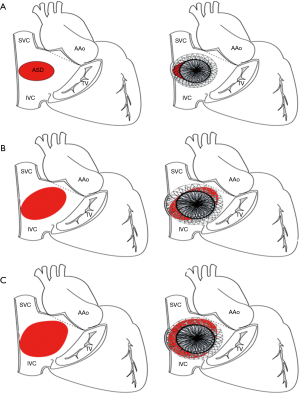
In case with posterior rim (Figure 1D) deficiency, the feasibility of device closure may be decided by the extent of rim deficiency (Figure 2). In the presence of rim deficiency in large area from IVC to posterior rim, the risk of device embolization is very high and this condition may preclude device closure.
In atrioventricular valve rim (Figure 1E) deficiency, encroachment of device onto the mitral and/or tricuspid valve is a potential problem. This is a concern especially in infants and young children because of the inherent design of Amplatzer-type devices which have a relatively larger disk-rim width in smaller devices. In case of device encroachment onto the valve, it is generally recommended not to implant a device. There is an extremely rare documented case of erosion on mitral valve (31).
Multiple defects
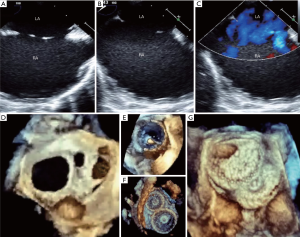
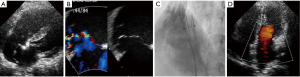
There are many challenges or considering factors when planning closure of multiple ASDs, including numbers/size of the defect, location/spatial relationship between the defects, properties of supporting rims or intervening septum as well as presence of septal aneurysm. Ultimately, understanding the accurate anatomy and properties of surrounding/intervening rims of multiple defects is the cornerstone of successful device closure. To overcome these problems, proper use of real time 3-dimensional (RT3D) echocardiography may be helpful (32,33). RT3D echocardiography enables visualization of the wide ranged septum in a single view in the echocardiography and provides instantaneous understanding of the anatomy as well as identification of complex morphology and spatial relationship between multiple defects (Figure 3). Temporary balloon occlusion test may also be useful to investigate compliance of surrounding rims and intervening septum, as well as to predict changes of the defects and rims after device implantation. Also, a careful observation of fluoroscopic images with balloon sizing may provide additional information on the spatial relationship between the defects and intervening septum (Figure 4). In case of device closure of multiple defects using multiple devices, the optimal combination of devices based on the comprehensive information from RT3D echocardiography and balloon occlusion test is required to prevent unfavorable interference between multiple devices (Figure 5). Usually, a small additional defect adjacent to a larger defect (<7 mm in distance) can also be closed by implantation of a single device in the major defect (34). When the additional defect is also sizable or defects are in distance each other, use of multiple devices is required. For multi-fenestrated defects with a large septal aneurysm, patch-like closure using a non-self-centering device may be a good option (Figure 6).
Currently available devices for ASD closure (Figure 7)
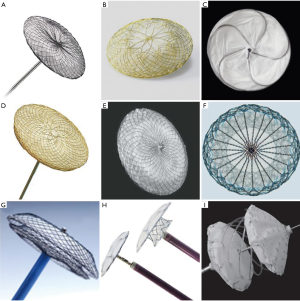
ASO (Figure 7A, Table 2)
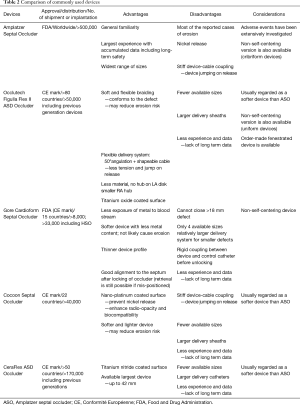
Full table
The ASO is the first self-expanding double-disk device with central connecting waist composed of nitinol-wire-mesh. The disks and waist were sewn with a Dacron patch to promote complete occlusion and endothelialization. The device allowed easy and straightforward deployment due to its self-centering, repositionable, and recapturable characteristics. The ASO solved many limitations of previous devices such as the non-negligible rate of residual shunt and frame fracture, and is regarded as a prototype device for many newer devices. The device size is determined by the waist size; there are 27 device sizes from 4 to 40 mm (in 1-mm increments between 4 to 20 mm and 2-mm increments between 20 to 40 mm). The corresponded delivery sheath sizes range from 6 to 12 French (Fr).
Initial study showed excellent success rate of 95.7% and low major and total adverse effect of 1.6% and 7.2% respectively (3). The subsequent MAGIC study and IMPACT registry reported compatible success and complication (35,36). Largest experience has been accumulated in ASO, but the risk of erosion has been issued (37) since it can be fatal and may occur delayed. The exact rate is still unclear, but the estimated rate is about 0.1 to 0.3 (38). Reported risk factors include aortic rim deficiency, superiorly located ASD, over-sizing of device, septal malalignment, dynamic ASD and lower patient weight: device size ratio (11,30,37-40), and the most probable mechanism is abrasion due to seesaw movement according to the cardiac cycle (37), although the exact mechanism is unclear or not known.
Recommendations to avoid erosion should be followed (11) and comprehensive evaluation by echocardiography during follow-up is warranted (30,40).
The ASO “cribriform” is a specially designed non-self-centering device for multi-fenestrated defects with 4 available sizes from 18 to 40 mm.
Occlutech Figulla Flex II ASD Occluder (Figure 7B)
Figulla Flex II ASD Occluder (FSO, Occlutech GmbH, Jena, Germany) is the third generation Occlutech device for ASD closure with a flexible titanium-oxide coated nitinol-mesh and double-disk design similar to ASO. FSO has minimized metal contents especially in the LA disk and no clamping hub on the LA disk, which may provide more flexible and less traumatic feature. The FSO has a distinct release mechanism resembling a bioptome which enables flexible movement between the device and delivery cable. The delivery cable is also shapeable for a better alignment between the device and septum.
There are 20 device size options from 4 to 40 mm (1–1.5-mm increments between 4 and 21 mm, 3-mm increments between 21 and 39 mm, and 40 mm). Retrospective comparison studies showed a compatible efficacy and safety comparing to ASO (41,42). The largest study with FSO, the IRFACODE study, showed a 98% success rate in 1395 patients and a 97.3% complete closure rate at 1 year (43). The major complication rate was minimal (n=8, <1%). Erosion has not yet been reported with this device. The disadvantages are its relatively limited size options (20 vs. 27 sizes in ASO) and requirement of a slightly larger delivery sheath than in ASO devices.
A fenestrated device for closure of ASD with pulmonary hypertension or risk of masked left ventricular restriction is available on an order-made basis.
GSO (Figure 7C)
The GSO is a non-self-centering double disk device composed of platinum filled nitinol wire framework covered with an expanded polytetrafluoroethylene (ePTFE) membrane to promote rapid endothelialization. The GSO is preloaded as a package of the device and whole delivery system. The delivery system has improved for easier delivery and position compared to that of the previous Helex Septal Occluder (HSO). There are 4 device sizes from 15 to 30 mm with 5-mm increments. It is generally recommended that the device size should be more than twice the defect size and the GSO is not suitable for defects over 18 mm due to the non-self-centering feature. Owing to its flexibility with minimal metal content which may prevent erosion, the device is preferred to close smaller defects especially with aortic rim deficiency or those in small children (44). Clinical studies verified efficacy and safety of this device in closing ASDs with various morphologies such as aortic rim deficiency, septal aneurysm and multiple defects in selected patients with feasible anatomy (12,13,45).
The Gore Cardioform ASD Occluder (GAO) is a self-centering version of GSO which consists of a helical nitinol-wire frame covered with ePTFE to treat larger ASDs. The available sizes are 27, 32, 37, 44, and 48 mm and designed to treat defects from 8 to 35 mm. Although this device is not commercially available yet, the initial clinical result showed the safety and efficacy of the GAO (46). The risk factor for procedural failure in GAO implantation was a larger defect size, especially a size larger than 27 mm by balloon sizing.
CeraFlex ASD Occluder (Figure 7D)
CeraFlex (LifeTech Scientific Co., Shenzhen, China), the 4th generation ASD device from LifeTech, is a nitinol-wire-mesh device coated with titanium nitride. This device is preloaded as a package and has a similar feature to other nitinol-wire-mesh devices such as self-expanding property and double-disk design with central waist. Ceraflex has couple of shared features with FSO; a coated surface of nitinol-wire, flexible connection between the device and delivery cable, and no hub on LA disk. A total of 19 sizes are available from 6 to 42 mm with 2-mm increments, and the delivery sheath sizes range from 8 to 14 Fr. A comparative study between Ceraflex and ASO showed comparable success rate, safety and efficacy (47). The disadvantages are its relatively limited size options (19 vs. 27 sizes in ASO) and the requirement of a slightly larger delivery sheath than with ASO devices.
Cocoon Septal Occluder (Figure 7E)
The Cocoon Septal Occluder (Vascular Innovations Co., Nonthaburi, Thailand) is a self-expanding double-disk device consists of a nano-platinum coated nitinol device filled with polypropylene fabric. The device is quite similar to ASO in terms of device design except for the nano-platinum-coated surface which prevents nickel release to the blood stream (48), promotes biocompatibility and enhances radio-opacity on fluoroscopy. There are 17 device options ranging from 8 to 40 mm with 2-mm increments and the delivery sheath sizes range from 7 to 14 Fr. European multicenter study reported an excellent result with procedural success rate of 100% and no complication in 92 patients (49). This device has been described as softest and lightest currently available device with less metal-to-septum ratio than other devices (49). The disadvantages are its relatively limited size options (17 vs. 27 sizes in ASO) and the requirement of a slightly larger delivery sheath than with ASO devices.
Nit-Occlud ASD-R (Figure 7F)
The Nit-Occlud ASD-R (NOA-R) (pfm Medical, Cologne, Germany) is a double-disk, self-expandable, self-centering device; however, the device characteristics are quite different from other nitrol-mesh devices. NOA-R has reduced amount of metal on the left atrial disk without clamping or screwing hub on either side of the atrial disks and has a “reverse configuration” of the single-nitinol-layer on the LA disk (the “R” on the product name indicates this). These characteristics may allow more flexible and conformable device positioning on the septum. The release mechanism is unique as it is “snare-like,” which includes a central locking wire and a pusher with a distal wire noose (50). A total of 12 sizes are available from 8 to 30 mm with 2-mm increments, and the delivery sheath sizes range from 8 to 14 Fr.
A multicenter study showed a 98.6% (73/74 patients) success rate with one case of complete heart block (50). Device retrieval is more difficult because of the no-hub design (50); however, retrieval of the device may be feasible using a special technique (snare and wire technique) with an oversized sheath (51). Two cases of erosion including one lethal case were reported (52,53). The disadvantages (are its relatively limited size options (12 vs. 27 sizes in ASO), and the requirement of a slightly larger delivery sheath than with ASO devices and inability to close larger defects.
Cardi-O-Fix Septal Occluder (Figure 7G)
The Cardi-O-Fix septal occluder (Starway Medical Technology, Beijing, China) is a self expandible double disk design device consists of nitinol wire mesh filled with polyester fabric sewn to waist and each disk. This device is structurally similar to the ASD, however this device has two different versions of products with or without clamping hub on the LA disk. A total of 27 sizes are available from 4 to 40 mm (1-mm increment between 4 to 20 mm and 2-mm increment between 20 to 40 mm), and the delivery sheath sizes range from 7 to 14 Fr. A comparative study between this device and ASO showed comparable outcome and lower cost with Cardi-O-Fix device (US$ 4,100 vs. US$ 5,900, P<0.001) (54). The disadvantages may include less experience/data with this device and the requirement of a slightly larger delivery sheath than with ASO.
Ultracept II ASD Occluder (Figure 7H)
The last generation of CARDIA ASD closure device, the Ultracept II ASD Occluder (Cardia, Eagan, MN, USA) is a double-round disk, self-centering, low profile device. It has a nitinol frame with a polyvinyl alcohol (PVA) coating to reduce thrombus formation. A total of 15 sizes are available from 6 to 34 mm with 2-mm increments, and the delivery sheath sizes range from 9 to 11 Fr. Several cases of PVA-membrane perforation or degradation were reported (55-57), which were also reported with the previous generation device (58). Despite comprehensive investigations on the PVA membrane, the mechanism of degradation has not been identified. Some authors emphasized that the interventionist should be aware of this rare complication (57).
Carag Bioresorbable Septal occluder (Figure 7I)
Carag Bioresorbable Septal Occluder (CBRO) (CARAG AG, Baar, Switzerland) is a self-centering, double disk device without any metal framework, composed of poly lactic-co-glycolic acid (59). Endothelialization of the device seems to be completed within 3 months, while the device usually starts to be resorbed after 6 months and completely resolved within 2 years. CBRO has 3 size options: small for 4–12 mm defects, intermediate for 13–20 mm, and large for 21–25 mm. Preliminary data showed an excellent efficacy of CBRO with successful outcome in all ten patients, 4 with ASD and 6 with patent foramen ovale (60).
Conclusions
Transcatheter closure of secundum ASD is the treatment of choice in most patients with feasible anatomy. An operator should be familiar with principles of the procedure, solutions for procedural difficulty, cautions to avoid complications and detailed knowledge on the available equipments to promote safety and efficacy of this versatile therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002;39:1890-900. [Crossref] [PubMed]
- Cowley CG, Lloyd TR, Bove EL, et al. Comparison of results of closure of secundum atrial septal defect by surgery versus Amplatzer septal occluder. Am J Cardiol 2001;88:589-91. [Crossref] [PubMed]
- Du ZD, Hijazi ZM, Kleinman CS, et al. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J Am Coll Cardiol 2002;39:1836-44. [Crossref] [PubMed]
- Al-Qbandi M, Cao QL, Hijazi ZM. Atrial septal defect: Amplazter-type ASD Occluders. In: Sievert H, Qureshi SA, Wilson N, et al. editors. Interventions in structural, valvular, and congenital heart disease. 2nd ed. CRC Press, 2015:437-47.
- Praz F, Wahl A, Schmutz M, et al. Safety, feasibility, and long-term results of percutaneous closure of atrial septal defects using the amplatzer septal occluder without periprocedural echocardiography. J Invasive Cardiol 2015;27:157-62. [PubMed]
- Ewert P, Berger F, Daehnert I, et al. Transcatheter closure of atrial septal defects without fluoroscopy: feasibility of a new method. Circulation 2000;101:847-9. [Crossref] [PubMed]
- Tzifa A, Gordon J, Tibby SM, et al. Transcatheter atrial septal defect closure guided by colour flow Doppler. Int J Cardiol 2011;149:299-303. [Crossref] [PubMed]
- Baruteau AE, Hascoet S, Fraisse A. Transthoracic echocardiography is a safe alternative for assessment and guidance of transcatheter closure of secundum atrial septal defect in children. J Thorac Dis 2017;9:1247-56. [Crossref] [PubMed]
- Wang JK, Tsai SK, Lin SM, et al. Transcatheter closure of atrial septal defect without balloon sizing. Catheter Cardiovasc Interv 2008;71:214-21. [Crossref] [PubMed]
- Kazmouz S, Kenny D, Cao QL, et al. Transcatheter closure of secundum atrial septal defects. J Invasive Cardiol 2013;25:257-64. [PubMed]
- Amin Z, Hijazi ZM, Bass JL, et al. Erosion of Amplatzer septal occluder device after closure of secundum atrial septal defects: review of registry of complications and recommendations to minimize future risk. Catheter Cardiovasc Interv 2004;63:496-502. [Crossref] [PubMed]
- Søndergaard L, Loh PH, Franzen O, et al. The first clinical experience with the new GORE(R) septal occluder (GSO). EuroIntervention 2013;9:959-63. [Crossref] [PubMed]
- Smith B, Thomson J, Crossland D, et al. UK multicenter experience using the Gore septal occluder (GSO(TM)) for atrial septal defect closure in children and adults. Catheter Cardiovasc Interv 2014;83:581-6. [Crossref] [PubMed]
- Varma C, Benson LN, Silversides C, et al. Outcomes and alternative techniques for device closure of the large secundum atrial septal defect. Catheter Cardiovasc Interv 2004;61:131-9. [Crossref] [PubMed]
- Harper RW, Mottram PM, McGaw DJ. Closure of secundum atrial septal defects with the Amplatzer septal occluder device: techniques and problems. Catheter Cardiovasc Interv 2002;57:508-24. [Crossref] [PubMed]
- Spies C, Boosfeld C, Schrader R. A modified Cook sheath for closure of a large secundum atrial septal defect. Catheter Cardiovasc Interv 2007;70:286-9. [Crossref] [PubMed]
- Kannan BR, Francis E, Sivakumar K, et al. Transcatheter closure of very large (>or= 25 mm) atrial septal defects using the Amplatzer septal occluder. Catheter Cardiovasc Interv 2003;59:522-7. [Crossref] [PubMed]
- Wahab HA, Bairam AR, Cao QL, et al. Novel technique to prevent prolapse of the Amplatzer septal occluder through large atrial septal defect. Catheter Cardiovasc Interv 2003;60:543-5. [Crossref] [PubMed]
- Dalvi BV, Pinto RJ, Gupta A. New technique for device closure of large atrial septal defects. Catheter Cardiovasc Interv 2005;64:102-7. [Crossref] [PubMed]
- Pillai AA, Rangaswamy Balasubramanian V, Selvaraj R, et al. Utility of balloon assisted technique in trans catheter closure of very large (>/=35 mm) atrial septal defects. Cardiovasc Diagn Ther 2014;4:21-7. [PubMed]
- Narin N, Baykan A, Argun M, et al. New modified balloon-assisted technique to provide appropriate deployment in the closure of large secundum atrial septal defect using amplatzer septal occluder in children. J Invasive Cardiol 2014;26:597-602. [PubMed]
- Kutty S, Asnes JD, Srinath G, et al. Use of a straight, side-hole delivery sheath for improved delivery of Amplatzer ASD occluder. Catheter Cardiovasc Interv 2007;69:15-20. [Crossref] [PubMed]
- Djer MM, Ramadhina NN, Idris NS, et al. Transcatheter closure of atrial septal defects in adolescents and adults: technique and difficulties. Acta Med Indones 2013;45:180-6. [PubMed]
- Fu YC, Cao QL, Hijazi ZM. Device closure of large atrial septal defects: technical considerations. J Cardiovasc Med (Hagerstown) 2007;8:30-3. [Crossref] [PubMed]
- Podnar T, Martanovic P, Gavora P, et al. Morphological variations of secundum-type atrial septal defects: feasibility for percutaneous closure using Amplatzer septal occluders. Catheter Cardiovasc Interv 2001;53:386-91. [Crossref] [PubMed]
- Mathewson JW, Bichell D, Rothman A, et al. Absent posteroinferior and anterosuperior atrial septal defect rims: Factors affecting nonsurgical closure of large secundum defects using the Amplatzer occluder. J Am Soc Echocardiogr 2004;17:62-9. [Crossref] [PubMed]
- Koenig P, Cao QL, Heitschmidt M, et al. Role of intracardiac echocardiographic guidance in transcatheter closure of atrial septal defects and patent foramen ovale using the Amplatzer device. J Interv Cardiol 2003;16:51-62. [Crossref] [PubMed]
- Remadevi KS, Francis E, Kumar RK. Catheter closure of atrial septal defects with deficient inferior vena cava rim under transesophageal echo guidance. Catheter Cardiovasc Interv 2009;73:90-6. [Crossref] [PubMed]
- Kammache I, Mancini J, Ovaert C, et al. Feasibility of transcatheter closure in unselected patients with secundum atrial septal defect, using Amplatzer devices and a modified sizing balloon technique. Catheter Cardiovasc Interv 2011;78:665-74. [Crossref] [PubMed]
- Amin Z. Echocardiographic predictors of cardiac erosion after Amplatzer septal occluder placement. Catheter Cardiovasc Interv 2014;83:84-92. [Crossref] [PubMed]
- Santoro G, Bigazzi MC, Lacono C, et al. Transcatheter closure of complex atrial septal defects: feasibility and mid-term results. J Cardiovasc Med (Hagerstown) 2006;7:176-81. [Crossref] [PubMed]
- Johri AM, Witzke C, Solis J, et al. Real-time three-dimensional transesophageal echocardiography in patients with secundum atrial septal defects: outcomes following transcatheter closure. J Am Soc Echocardiogr 2011;24:431-7. [Crossref] [PubMed]
- Silvestry FE, Cohen MS, Armsby LB, et al. Guidelines for the Echocardiographic Assessment of Atrial Septal Defect and Patent Foramen Ovale: From the American Society of Echocardiography and Society for Cardiac Angiography and Interventions. J Am Soc Echocardiogr 2015;28:910-58. [Crossref] [PubMed]
- Szkutnik M, Masura J, Bialkowski J, et al. Transcatheter closure of double atrial septal defects with a single Amplatzer device. Catheter Cardiovasc Interv 2004;61:237-41. [Crossref] [PubMed]
- Everett AD, Jennings J, Sibinga E, et al. Community use of the amplatzer atrial septal defect occluder: results of the multicenter MAGIC atrial septal defect study. Pediatr Cardiol 2009;30:240-7. [Crossref] [PubMed]
- Moore JW, Vincent RN, Beekman RH 3rd, et al. Procedural results and safety of common interventional procedures in congenital heart disease: initial report from the National Cardiovascular Data Registry. J Am Coll Cardiol 2014;64:2439-51. [Crossref] [PubMed]
- Diab K, Kenny D, Hijazi ZM. Erosions, erosions, and erosions! Device closure of atrial septal defects: how safe is safe? Catheter Cardiovasc Interv 2012;80:168-74. [Crossref] [PubMed]
- Crawford GB, Brindis RG, Krucoff MW, et al. Percutaneous atrial septal occluder devices and cardiac erosion: a review of the literature. Catheter Cardiovasc Interv 2012;80:157-67. [Crossref] [PubMed]
- McElhinney DB, Quartermain MD, Kenny D, et al. Relative Risk Factors for Cardiac Erosion Following Transcatheter Closure of Atrial Septal Defects: A Case-Control Study. Circulation 2016;133:1738-46. [Crossref] [PubMed]
- Mallula K, Amin Z. Recent changes in instructions for use for the Amplatzer atrial septal defect occluder: how to incorporate these changes while using transesophageal echocardiography or intracardiac echocardiography? Pediatr Cardiol 2012;33:995-1000. [Crossref] [PubMed]
- Godart F, Houeijeh A, Recher M, et al. Transcatheter closure of atrial septal defect with the Figulla((R)) ASD Occluder: a comparative study with the Amplatzer((R)) Septal Occluder. Arch Cardiovasc Dis 2015;108:57-63. [Crossref] [PubMed]
- Roymanee S, Promphan W, Tonklang N, et al. Comparison of the Occlutech (R) Figulla (R) septal occluder and Amplatzer (R) septal occluder for atrial septal defect device closure. Pediatr Cardiol 2015;36:935-41. [Crossref] [PubMed]
- Haas NA, Soetemann DB, Ates I, et al. Closure of Secundum Atrial Septal Defects by Using the Occlutech Occluder Devices in More Than 1300 Patients: The IRFACODE Project: A Retrospective Case Series. Catheter Cardiovasc Interv 2016;88:571-81. [Crossref] [PubMed]
- Grohmann J, Hohn R, Fleck T, et al. Transcatheter closure of atrial septal defects in children and adolescents: single-center experience with the GORE(R) septal occluder. Catheter Cardiovasc Interv 2014;84:E51-7. [Crossref] [PubMed]
- Grohmann J, Wildberg C, Zartner P, et al. Multicenter midterm follow-up results using the gore septal occluder for atrial septal defect closure in pediatric patients. Catheter Cardiovasc Interv 2017;89:E226-32. [Crossref] [PubMed]
- de Hemptinne Q, Horlick EM, Osten MD, et al. Initial clinical experience with the GORE(R) CARDIOFORM ASD occluder for transcatheter atrial septal defect closure. Catheter Cardiovasc Interv 2017;90:495-503. [Crossref] [PubMed]
- Astarcioglu MA, Kalcik M, Sen T, et al. Ceraflex versus Amplatzer occluder for secundum atrial septal defect closure. Multicenter clinical experience. Herz 2015;40 Suppl 2:146-50. [Crossref] [PubMed]
- Lertsapcharoen P, Khongphatthanayothin A, Srimahachota S, et al. Self-expanding platinum-coated nitinol devices for transcatheter closure of atrial septal defect: prevention of nickel release. J Invasive Cardiol 2008;20:279-83. [PubMed]
- Thanopoulos BD, Biasco L, Dardas P, et al. Catheter closure of atrial septal defects using the Cocoon septal occluder: preliminary results of a European multicenter study. Int J Cardiol 2014;177:418-22. [Crossref] [PubMed]
- Peirone A, Contreras A, Ferrero A, et al. Immediate and short-term outcomes after percutaneous atrial septal defect closure using the new nit-occlud ASD-R device. Catheter Cardiovasc Interv 2014;84:464-70. [Crossref] [PubMed]
- Granja MA, Peirone A, Barbosa JD, et al. ASD-R PFM device. In: Sievert H, Qureshi SA, Wilson N, et al. editors. Interventions in Structural, Valvular and Congenital Heart Disease. 2nd ed. Boca Raton: CRC Press, 2015;Chap 53:463-8.
- Bulut MO, Yucel IK, Kucuk M, et al. Initial Experience with the Nit-Occlud ASD-R: Short-Term Results. Pediatr Cardiol 2016;37:1258-65. [Crossref] [PubMed]
- Kister T, Dahnert I, Lurz P. Fatal Erosion Atrial Septal Defect Device. Catheter Cardiovasc Interv 2016;87:951-4. [Crossref] [PubMed]
- Saritas T, Kaya MG, Lam YY, et al. A comparative study of Cardi-O-Fix septal occluder versus Amplatzer septal occluder in percutaneous closure of secundum atrial septal defects. Catheter Cardiovasc Interv 2013;82:116-21. [Crossref] [PubMed]
- Ramoğlu MG, Ucar T, Tutar E. Early malfunction of polyvinyl alcohol membrane of septal occluder. Catheter Cardiovasc Interv 2016;87:E151-3. [Crossref] [PubMed]
- Bozyel S, Sahin T, Dervis E, et al. A massive left-to-right shunt due to delayed spontaneous perforation of polyvinyl alcohol membrane of atrial septal occluder. Turk Kardiyol Dern Ars 2017;45:541-4. [PubMed]
- Labombarda F, Roule V, Beygui F. Delayed spontaneous perforation of polyvinyl alcohol membrane-Covered atrial septal defect closure devices. Catheter Cardiovasc Interv 2017;89:E141-4. [Crossref] [PubMed]
- Bartel T, Bonaros N, Muller S. Device failure weeks to months after transcatheter closure of secundum type atrial septal defects. Heart 2010;96:1603. [Crossref] [PubMed]
- Nassif M, Abdelghani M, Bouma BJ, et al. Historical developments of atrial septal defect closure devices: what we learn from the past. Expert Rev Med Devices 2016;13:555-68. [Crossref] [PubMed]
- Sievert H, Söderberg B, Mellmann A, et al. First human use and intermediate follow-up of a septal occluder with a bioresorbable framework. EuroPCR, 2015;Paris.


