The comparison of predictive factors regarding prognoses and invasion of thymic neuroendocrine tumors preoperatively and postoperatively
Introduction
Thymic neuroendocrine tumors (TNT) are extremely rare. In all kinds of anterior mediastinal masses, the incidence of TNT is less than 5% (1,2). Previous research based on Surveillance, Epidemiology and End Results database (SEER) demonstrated TNT possessed only 0.4% of all neuroendocrine tumors (NETs) in the body and occurred in 0.02/100,000 population per year (3). However, the incidence of TNT has increased recently (4). In most body parts, the malignancy of carcinoid is significantly lower than carcinoma (5). However, the thymus carcinoid has a higher degree of malignancy than thymoma and thymus carcinoma (2). Five- and 10-year survival rates are 56% and 26%, which are significantly worse than thymoma (2).
Previous studies reported male to female ratio of TNT incidence as approximately 3:1, with an average age of 54 years (5,6). Two major factors attribute to symptoms of TNT. First, tumors invade or compress surrounding or adjacent structures. Second, TNT could secrete bioactive amines excessively and cause carcinoid syndrome, including wheezing, skin flushing, diarrhea, and fibrotic valvular heart disease (7,8). In addition to this, adrenocorticotropic hormone (ACTH) and corticotropin-releasing hormone (CRH) are the most commonly secreted by TNT to cause the pertinent endocrine symptoms (2,6,8).
The most common clinical staging system for TNT is Masaoka stage classification (2). Besides, three grade-groups have been categorized by histological classification, which are typical carcinoid tumor (CT), atypical carcinoid tumor (AT), and neuroendocrine carcinoma (NT) (6,9).
With the differences in gender, clinical stage, and histological grade, TNT has revealed significantly different characteristics, prognoses, and metastatic rate. According to various patients and tumors characteristics, the treatments of TNT have been enormously controversial (1-5), which includes procedures with or without adjuvant radiotherapy/chemotherapy/targeted-therapy at each clinical stage, type of regimens of chemotherapy/targeted therapy, and so on. Studies to focus on these issues have been rare and lack clear demonstration. Therefore, we attempted to identify the prognostic factors of TNT preoperatively and postoperatively and tried to explore the critical reasons that caused the differences in prognostic factors between the preoperative and postoperative groups. Besides, we evaluated the effectiveness of adjuvant treatments postoperatively. Finally, we aimed at constructing the relationship between the possibility of TNT invasiveness and tumor histology.
Methods
Patients diagnosed with TNT between 1998 and 2014 from SEER were enrolled. The inclusion criteria were: (I) patients diagnosed with TNT; (II) TNT were defined as CT, AT, and NT, according to histological classification; (III) patients information included geographic location, tumor characteristics, therapeutic regimens, follow-up information, and so on, and were recorded in detail. Patients were excluded if: (I) they had a history of other tumors; (II) had incomplete clinical stage information, histological grade, therapeutic and follow-up. Patients parameters retrieved from SEER included: (I) age at diagnosis; (II) gender; (III) histological grade; (IV) SEER summary stage; (V) metastatic status at the time of diagnosis; (VI) therapeutic regimens; (VII) whether or not the patient received chemotherapy; (VIII) whether or not the patient received radiotherapy, and (IX) time from diagnosis until the last contact. The SEER summary stage was defined in the same manner as Masaoka stage (10) (Masaoka stage I: L; Masaoka stage II–III: R; Masaoka stage IV: D). The prognostic factors such as resected marginal status, regimens, dose, toxicity, and a side-effect of adjuvant therapies were recorded. Information on recurrent sites was not included in the SEER. Hence, they were not analyzed in this study.
Statistical analysis was performed by SPSS software, version 23.0 (SPSS Inc., Chicago, USA). Student t-test, chi-square test or Fisher exact test were performed to analyze continuous and nominal data variables. Kaplan-Meier method was used to evaluate the overall survival (OS) of patients. Univariate analysis was performed by employing log-rank test to predict relations between parameters and OS. Cox proportional hazards regression method was performed to identify predictors of OS. Logistic regression model was used to estimate the correlation between tumor histology and the possibility of metastasis. OS was calculated from the date of diagnosis to death from any cause. Patients who were alive at last contact were censored at the time. A two-tailed P value <0.05 was considered statistically significant. The hazard ratio (HR) and odds ratio (OR) were presented with 95% confidence interval (CI).
Results
Patients’ baseline and characteristics
A total of 173 TNT patients were enrolled in this study. Our eligibility criteria selected patients between 1998 and 2014 with the desired dataset for inclusion in our retrospective cohort analysis with fewer survival variables. We were able to identify a group of patients in this period who shared common features based on our inclusion/exclusion criteria to identify relationships between the characteristics of the study population such as tracking their original hospital referral, demographic variables, diagnosis, surgical excision, radiotherapy, chemotherapy, hormonal therapy outcomes, and follow-up time. Additionally, we considered inclusion of detailed schemes of recordings of radiotherapy, and whether delivery was neoadjuvant, adjuvant or intraoperative, whose additions to the SEER registry was not initiated until 1983 and were completed for all tumor types by 1998. Further, we excluded patients with a history of other known tumors. Thus, we retrieved a total of 173 patients, compared to Sullivan et al. [2017] (2) study which analyzed the same registry between 1988 and 2011, identifying 254 patients. Further, the SEER program, sponsored by the National Cancer Institute (NCI), does not make data on chemotherapy and hormone therapy publically available due to concerns about the completeness of the information. However, we were able to assess the use of chemotherapy by a specific request from the NCI.
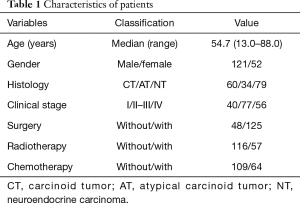
The baseline, characteristics, and therapeutic approaches are listed in Table 1. The average age of diagnosis was 54.7 years (range, 13.0–88.0 years). The ratio of males to females was approximately 2.5. According to the histological classification, patients were divided into CT, AT, NT three grade-groups, which had 60, 34, and 79 patients, respectively. According to Masaoka clinical stage, patients were divided into three stage-groups (stage I, stage II–III, and stage IV), which comprised 40, 77, and 56 patients, respectively. Patients who received surgery, radiotherapy, and chemotherapy, were 125, 57, and 64, respectively.
Full table
OS analysis
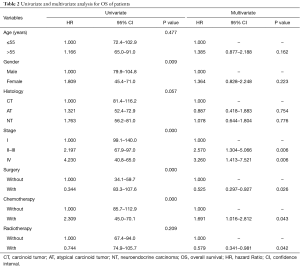
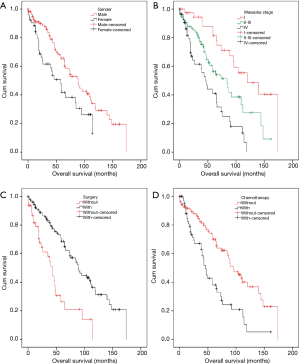
To identify the factors affecting the survival of TNT patients, OS was analyzed (see Table 2). Based on our univariate analysis, diagnostic age, histological grade, and radiotherapy did not correlate with OS. Patients were divided into two age-groups (≤55 vs. >55 years), based on averaging of their diagnostic age. According to log-rank test, no significant difference of OS between the two age-groups was observed. Besides, no difference of OS was observed between patients with and without radiotherapy. However, significant better OS was observed in male than female patients (OS rate: 54.4% vs. 48.6%, OS time: 103.4 vs. 63.7 months, P=0.009) (Figure 1A). Upgrading of clinical stage (stage I vs. II–III vs. IV) significantly predicted worse OS (OS rate: 60.0% vs. 51.9% vs. 39.3%, OS time: 119.5 vs. 82.4 vs. 52.9 months, P<0.001) (Figure 1B). Surgically treated patients had significantly better OS, compared with patients who did not receive surgery (OS rate: 52.8% vs. 41.7%, OS time: 95.4 vs. 46.9 months, P<0.001) (Figure 1C). Surgery was the most important approach to complete cure of the diseases (2-6). Chemotherapy treatment significantly decreased OS (OS rate: 56.9% vs. 37.5%, OS time: 99.3 vs. 57.6 months, P<0.001) (Figure 1D). Overall, our results indicated that gender, clinical stage, surgical treatment, and chemotherapy could predict prognoses of TNT patients.
Full table
Cox regression model was used to evaluate all the variables in a univariate analysis. Multivariate analysis showed that the predictive prognostic factors were clinical stage (II–III vs. I: HR, 2.570, 95% CI: 1.304–5.066, P=0.006; IV vs. I: HR, 3.260, 95% CI: 1.413–7.521, P=0.006), surgery (with vs. without: HR, 0.525, 95% CI: 0.297–0.927, P=0.026), chemotherapy (with vs. without: HR, 1.691, 95% CI: 1.016–2.812, P=0.043), and radiotherapy (with vs. without: HR: 0.579, 95% CI: 0.341–0.981, P=0.042) (Table 2). These results revealed that clinical stage, surgery, and adjuvant therapies were independent risk factors for OS. The local clinical stage, with surgical treatment, radiotherapeutic treatment, but without chemotherapeutic alone treatment, significantly predicted better OS.
Correlation with invasive possibility and histology
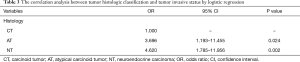
The logistic regression model was employed to analyze the correlation between the histology and metastatic possibility of the TNT patients. With upgrading histological grade, the possibility of tumor invasion into mediastinal structures, distant lymph nodes or distant organs was significantly increased (AT vs. CT: OR, 3.696, 95% CI: 1.193–11.455, P=0.024; NT vs. CT: OR, 4.620; 95% CI: 1.785–11.956; P=0.002) (Table 3). These results showed that CT patients experienced metastasis at a lower rate than AT and NT patients (an increase of 3.696 and 4.620 times, respectively). The metastatic possibility and TNT histology had a definite correlation.
Full table
OS analysis for surgically treated patients
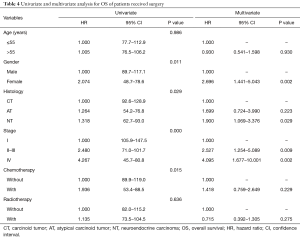
Since surgical treatment is the only approach to complete cure of the disease, and it could increase TNT patients’ OS significantly (2-6), we excluded the patients who did not receive surgery (see Table 4). The prognostic factors were analyzed by univariate and multivariate analysis on the remaining 125 surgically treated patients.
Full table
Based on univariate analysis, age at diagnosis and radiotherapy did not correlate with patients’ OS, while, gender, histological grade, clinical stage, and chemotherapy, significantly affected OS. Females had significantly worse OS than males (males vs. females, OS rate: 54.4% vs. 48.6%, OS time: 103.4 vs. 63.7 months, P=0.011). With upgrading histological grade (CT & AT vs. NT, OS rate: 57.5% vs. 46.2%, OS time: 105.4 vs. 77.8 months, P=0.050) and upgrading clinical stage (stage I vs. II–III vs. IV, OS rate: 61.8% vs. 52.3% vs. 42.3%, OS time: 126.6 vs. 86.3 vs. 66.3 months, P<0.001), patients’ OS decreased significantly. Patients with chemotherapy showed significantly poorer OS than patients who did not receive chemotherapy (with vs. without, OS rate: 44.7% vs. 56.3%, OS time: 71.0 vs. 104.4 months, P=0.015) (Table 4). Based on multivariate analysis, Cox regression analysis was performed. The results revealed that gender (female vs. male, HR: 2.696, 95% CI: 1.441–5.043, P=0.002), histological grade (NT vs. CT, HR: 1.900, 95% CI: 1.069–3.376; P=0.029) and clinical stage (stage II–III vs. I, HR: 2.527, 95% CI: 1.254–5.089, P=0.009; stage IV vs. I, HR: 4.095, 95% CI: 1.677–10.001, P=0.002) were independent risk factors for OS. These results showed that patients’ gender, TNT tumor characteristics histological grade, and the clinical stage could predict prognoses for surgically treated patients. Compared with the preoperative independent risk factors, gender became a postoperative risk factor, while adjuvant therapies disappeared.
Stratification analysis for effectiveness of adjuvant therapies
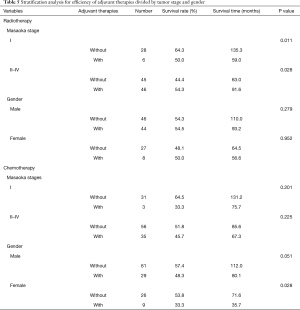
Therapeutic regimens could indeed influence patients’ OS. According to patients’ and tumors’ characteristics, patients were divided into subgroups, and the effectiveness of adjuvant therapies was analyzed. Our present results revealed no relationship of adjuvant effectiveness to patients’ age and tumor histology, whereas different clinical stages and genders influenced the effectiveness of adjuvant chemotherapy and radiotherapy (Table 5).
Full table
In Masaoka stage I, TNT had complete capsula. However, in the advanced stage, TNT invaded capsula, surrounding structures, distant lymph nodes, and distant organs were observed. Patients were stratified into two stage-groups by different invasive status. Stage I patients without radiotherapy had significantly better OS than the others with radiotherapy (without vs. with, OS rate: 64.3% vs. 50.0%, OS time: 135.3 vs. 59.0 months, P=0.011) (Figure 2A). In the advanced stage (stage II–IV), patients without radiotherapy had significantly worse OS than the patients with radiotherapy (without vs. with, OS rate: 44.4% vs. 54.3%, OS time: 63.0 vs. 91.6 months, P=0.028) (Figure 2B). Our data revealed that adjuvant radiotherapy predicted worse prognoses for Masaoka stage I patients postoperatively. However, it indicated better prognoses for postoperative, advanced stages patients.
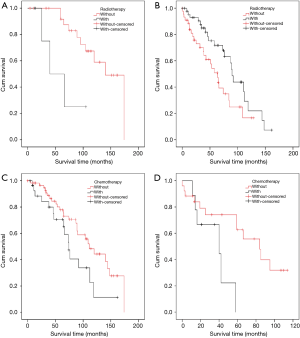
Male patients without chemotherapy had marginal better OS than male patients who received chemotherapy (without vs. with, OS rate: 57.4% vs. 48.3%, OS time: 112.0 vs. 80.1 months, P=0.051) (Figure 2C). Female patients without chemotherapeutic treatment had significantly better OS rate with a longer duration than females who received chemotherapy (without vs. with, OS rate: 53.8% vs. 33.3%, OS time: 71.6 vs. 35.7 months, P=0.028) (Figure 2D). These results suggested that chemotherapy was a negative factor in patients’ OS, especially, in females.
Discussion
In the present study, 173 TNT patients were enrolled in SEER. We analyzed the predictive factors of prognoses and built the relationship between histology and the possibility of invasive TNT. Since with TNT, excess hormones and bioactive amines are secreted to cause carcinoid syndromes and relevant symptoms, the diseases might be diagnosed at a relatively early stage (1,8). Surgical treatment occurs in high proportion in therapeutic regimens of TNT and has had relatively better outcomes (2,6). Therefore, we excluded non-surgical treated patients, estimated prognostic factors of TNT postoperatively, and compared the preoperative and postoperative prognostic factors. Additionally, we evaluated the effectiveness of adjuvant treatments based on different patients’ and tumor characteristics.
First, TNT’s prognostic factors identified in this study demonstrated that female patients in advanced clinical stages, without surgery, and with chemotherapy were predicted to have significantly worse prognosis (P<0.01, for all). Multivariate analysis revealed that independent risk factors for OS were a clinical stage, surgery, chemotherapy, and radiotherapy.
Second, gender was a preoperative factor of prognoses in univariate analysis, while no significant difference of OS was observed between male and female patients in the multivariate analysis. As we all know, different health attitudes and behaviors between male and female patients would affect patients’ OS profoundly (11). Additionally, chi-square test revealed that the proportion of radiotherapeutic treated patients in male-group was significantly higher than female-group (male vs. female: 35.3% vs. 19.9%, P=0.030). We speculated that gender and radiotherapy factors could influence OS simultaneously. Besides, the differences in TNT survival by gender probability, NET in other organs, such as esophagus, colon, thyroid, pancreas, and so on, has revealed carcinoid syndrome more frequently in females than in males, and reports have shown that patients with carcinoid syndrome had significantly shorter OS than those without the condition (8,11). Compared with male patients, females had a high incidence of secretion of CRH, ACTH, estrogen (EN) which could (I) enhance the degree of malignancy and the rate of development of TNT, (II) raise the possibility of females having a lower response to adjuvant chemotherapies (8,11-14). These factors probably caused female patients to be diagnosed in a relatively advanced stage than male and also disfavored the effectiveness of traditional adjuvant chemotherapies in the treatment of female patients, thus bringing about relatively worse OS in female than male patients.
Third, radiotherapy as an independent risk factor in the multivariate analysis could not be identified by univariate analysis. We concluded that the usage of radiotherapy in the advanced stage was significantly frequent than did those in the local stage (I vs. II–IV: 15.0% vs. 38.3%, P=0.019). Therefore, we could not identify from our univariate analysis the improvement of OS via adjuvant radiotherapy and could merely be identified by our multivariate analysis. What is more, the relevant information that explained the doubts such as the patients’ condition, the sequence of treatments, dose, and a side-effect of adjuvant regimens, evidently could not be acquired from SEER and were the significant limitations in this study.
Fourth, as we all know, surgery is the essential treatment regimen for TNT, as it is the only approach to cure TNT completely (2,5,6) and patients have had significantly better prognoses by surgical treatments than without surgery (3-6). Because of these, we utilized survival analysis to evaluate the OS of 125 TNT surgically treated patients, which identified the predictive factors of OS postoperatively. There have been limited studies which have focused on this topic previously (2,6,15-17). Sullivan reported that patients’ diagnostic age had no relation to survival (2), consistent with our findings. Sullivan et al., and Filosso et al., showed that gender did not influence patients’ survival as well (2,6). However, in our present study, survival rate and OS duration in female patients were significantly poorer than male patients postoperatively. As mentioned above, female TNT patients had worse prognoses because of the factors that affect survival, such as health attitudes, behaviors, influence of bioactive amines (EN, CRH, ACTH), and response to adjuvant therapies, are different between male and female patients (8,11-14). Postoperatively, gender was an independent risk factor for OS.
Fifth, the results showed histological grade, clinical stage, and chemotherapy also affected postoperative OS significantly. With histological grade and clinical stage upgrading, and the usage of adjuvant chemotherapy, TNT patients had significantly poorer OS postoperatively. Based on multivariate analysis, gender, histology and clinical stage were independent risk factors for postoperative prognoses. Compared with the preoperative prognostic factors, gender became an independent risk factor postoperatively. Female patients had significantly worse OS than male postoperatively. These findings should call surgeons attention to emphasize and standardize postoperative follow-up and treatments for female patients. These outcomes have made it critical to advocate for postoperative prognoses in TNT patients. The mechanism of gender disparities in OS will be researched in future studies.
Sixth, excepted for the above-mentioned factors, tumor size and resected marginal status could affect prognoses as well. Sullivan and Weksler (2017) showed the tumor size significantly affect TNT patients’ survival. However, it was not a significant variable affecting survival postoperatively (2). Gaur et al. showed that tumor size larger than 5 cm could significantly increase the possibility of TNT metastasis (3). Cardillo et al. showed that tumor size larger than 7 cm could significantly shorten postoperative survival of TNT patients (15). As our unpublished study concerning thymus tumors revealed that, with increasing tumor size, patients’ survival decreased significantly (detailed information is not shown). Besides, previous studies reported that complete resection significantly improved OS postoperatively (6,10,18,19), consistent with previous studies based on SEER (2,3). Information of resected marginal status could not be obtained from the SEER, posing an additional limitation in the present study as well. These issues will be clarified by our single-center data in the further research.
Seventh, radiotherapy had different effectiveness for TNT patients in different clinical stages, as reported by several previous studies (2,3,6,10,17). Gaur elucidated that patients who had a positive resected margin with radiotherapy had significant benefits. Radiotherapy was a positive prognostic factor for the advanced stage TNT (3). Therefore, to demonstrate the effectiveness of radiotherapy, we divided patients into two stage-groups. Radiotherapy was a significantly negative predictive factor for prognoses postoperatively in the local stage patients (with vs. without: 64.3% vs. 50.0%, 135.3 vs. 59.0 months, P=0.011). However, in the advanced stage, OS of patients with radiotherapy was better than patients without radiotherapy significantly (with vs. without: 44.4% vs. 54.3%, 63.0 vs. 91.6 months, P=0.028). Thus, we concluded that treating TNT patients with adjuvant radiotherapy in specific stages could promote OS. On the one hand, as TNT occurred microscopically, macroscopic invasion or metastasis, adjuvant radiotherapy could significantly increase patients’ OS postoperatively. On the other hand, if TNT were local, radiotherapy could decrease patients’ OS significantly. These results demonstrate that surgical finding and pathological results played important roles to assist surgeons in making postoperative radiotherapeutic regimens.
Eighth, as well as surgery and radiotherapy, adjuvant chemotherapy was a traditional regimen to treat malignant diseases. However, there were limited reports regarding the relationships between the effectiveness of chemotherapy and survival of TNT. As previous studies showed that patients received chemotherapy preoperatively could advocate the rate of complete resection (8,20). However, for the high rate of metastasis, chemotherapy was useless to advocate the rate of complete resection and promote their OS when metastases occur in patients (20). Our study was the first to reveal the effects of chemotherapy in the treatment of TNT in a large population analysis. In our univariate analysis, we found that patients with chemotherapy predicted significantly worse OS than the patients without chemotherapy, preoperatively and postoperatively (P<0.001 and P=0.015, respectively). Our multivariate analysis, however, showed no significant difference of OS between patients with or without chemotherapy. These findings showed that chemotherapy could indeed decrease patients’ prognoses, but it is not an independent risk factor for OS. Therefore, to demonstrate the effectiveness of chemotherapy, we divided patients into stage-groups and gender-groups respectively according to Masaoka stage and gender of patients. Regardless of tumor progression (with or without invasion). We found that patients treated with chemotherapy had worse OS than patients without chemotherapy. The discrepancies of OS had no statistic difference (P>0.05). However, it certainly showed that traditional chemotherapy decreased patients’ OS, and the negative effects of chemotherapy had no correlation with clinical stage of TNT. Interestingly, in the male-group, patients who received chemotherapy predicted marginal worse OS than patients without chemotherapy (P=0.051). However, OS rate and time of female patients with chemotherapy were significantly worse than the female patients without chemotherapy (33.3% vs. 53.8%, 35.7 vs. 71.6 months, P=0.028). Therefore, we concluded that chemotherapy was a negative factor for TNT patients’ OS, especially female patients. It revealed that traditional chemotherapy should not recommend in treating TNT patients, and should even be banned from treating female TNT patients. The finding that the response of traditional chemotherapy to treat TNT was unfavorable, in particular for female patients, calls for immediate research studies for suitable molecular targets and new medicines for chemotherapy and targeted therapy treatment of TNT.
Ninth, based on the histological classification of TNT, this study is the first to build the relationship between tumor histology and the possibility of TNT invasion. Previous studies concluded that increasing histological grade predicted poor survival (2,3) and increasing tumor size correlated with increasing metastatic rate (15). However, the correlation with tumor histology and the possibility of invasiveness could not be constructed. In this study, using logistic regression model, we found that AT and NT had a significantly higher risk to occur microscopic or macroscopic metastasis compared with CT (OR =3.696, 4.620; P=0.024, 0.002; respectively). The results represented compared with CT, showed an invasive possibility of AT and NT increased 3.696 and 4.620 times. Thus, AT and NT metastases could occur more frequently than CT, inducing worse prognoses. Thus, this should call surgeons and physicians attention to be alert of postoperative metastasis, even though there is the likelihood of patients of TNT to receive complete resection, after pathological diagnosis of AT or NT.
Finally, our results show that postoperative Masaoka stage I patients had poor prognosis from adjuvant chemotherapy, but advanced stages patients demonstrated better prognosis, it is not advisable to suggest adjuvant radiotherapy postoperatively on stage I TNT patients; whereas this combined treatment strategy could be recommended for patients in stage II–IV to improve OS.
Study limitations
SEER database, a large population retrospective database, inevitably, has some limitations. SEER does not include the data of the patients’ condition, surgical resection status, adjuvant therapeutic regimens, dose, toxicity, a side-effect of adjuvant-therapies, and the public display of chemotherapy data. These limitations restrict us to discuss the effectiveness of surgery and adjuvant therapies thoroughly. Furthermore, we were unable to compare results of complete versus incomplete resection and resection versus radiotherapy in this analysis because of lack of SEER data. This limitation might result from the fact that overall, most patients with locally advanced cancer also had metastasis and resection was usually not possible. Additionally, the issue of metastasis, their impact on outcomes, and extent of thymic surgery have remained controversial (2,6,21). Further, we were unable to include the presence of comorbidities, their associations with cancer progression, and mortality rates in our present analysis due to lack of information on comorbidity data such as diabetes, hypertension, and cardiovascular diseases in SEER registries (22,23).
Although SEER database has been used to demonstrate bronchial and thymic NETs in recent studies which analyzed patients who underwent surgical intervention, with cases involving distant or metastatic disease, it has been shown that surgical intervention could not play a role and hence, is rarely curable. Thus, the therapeutic options for metastatic/advanced NETs are limited mainly to palliate symptoms. Therefore, recommendations for patients with bronchial or thymic NETs are individualized treatments, weighing the risks and benefits of therapy. This has brought about inconsistencies of reports in the SEER registries. We are currently researching to refine appropriate methods of analyses in order to map variables that are likely to impact outcomes for future reports.
Unfortunately, certain cases of malignancy, such as gastric and thoracic cancers are diagnosed in a locally advanced tumor stage. Patients with these types of tumors usually demonstrate poor prognosis attributable to the frequent recurrences after primary resection with a curative objective, an observation which brought about the development of (neo) adjuvant treatment concepts. Although our analysis revealed that adjuvant therapies did not correlate with patients’ age and tumor histology, different clinical stages and genders influenced the effectiveness of adjuvant chemotherapy and radiotherapy, consistent to numerous reports (24-30). Cunningham et al. and Ychou et al. showed the advantage of perioperative chemotherapy over surgery alone in randomized trials (24,25). Schuhmacher and coworkers were able to show higher R0 resection rates in patients receiving neoadjuvant chemotherapy. Although this effect did not translate into prolonged OS, excellent survival rates were attained, compared to surgery alone (26).
As an extremely rare disease, the number of TNT patient is inadequate; the effectiveness of chemotherapy in each stage-group need be confirmed in future research. However, our analysis revealed some prognostic factors and prognosis-related factors, using the database which are clear, reasonable, and reliable.
Conclusions
TNT clinical stage, surgery, and adjuvant therapies are prognostic factors for TNT patients’ OS, while, gender, clinical stage and histological grade predict worse postoperative prognoses. Compared with male patients, female patients have significantly worse OS postoperatively. With upgrading histological grade and clinical stage, postoperative OS decreased significantly. Radiotherapy has significant benefit for the patients in clinical stage II–IV. However, it resulted in significantly worse prognoses for stage I patients. Chemotherapy significantly has a disadvantage for postoperative OS, especially, for female patients. Additionally, compared with CT patients, AT and NT patients have significantly high possibility for the occurrence of metastasis and invasion.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This article does not contain studies involving human participants or the use of experimental animals by any of the authors. Informed consent was obtained from each participant included in the study.
References
- Goto K, Kodama T, Matsuno Y, et al. Clinicopathologic and DNA cytometric analysis of carcinoid tumors of the thymus. Mod Pathol 2001;14:985-94. [Crossref] [PubMed]
- Sullivan JL, Weksler B. Neuroendocrine Tumors of the Thymus: Analysis of Factors Affecting Survival in 254 Patients. Ann Thorac Surg 2017;103:935-9. [Crossref] [PubMed]
- Gaur P, Leary C, Yao J. Thymic neuroendocrine tumors: a SEER database analysis of 160 patients. Ann Surg 2010;251:1117-21. [Crossref] [PubMed]
- Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. [Crossref] [PubMed]
- Chaer R, Massad MG, Evans A, et al. Primary neuroendocrine tumors of the thymus. Ann Thorac Surg 2002;74:1733-40. [Crossref] [PubMed]
- Filosso PL, Yao X, Ahmad U, et al. Outcome of primary neuroendocrine tumors of the thymus: a joint analysis of the International Thymic Malignancy Interest Group and the European Society of Thoracic Surgeons databases. J Thorac Cardiovasc Surg 2015;149:103-9.e2. [Crossref] [PubMed]
- Beaumont JL, Cella D, Phan AT, et al. Comparison of health-related quality of life in patients with neuroendocrine tumors with quality of life in the general US population. Pancreas 2012;41:461-6. [Crossref] [PubMed]
- Halperin DM, Shen C, Dasari A, et al. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: a population-based study. Lancet Oncol 2017;18:525-34. [Crossref] [PubMed]
- Travis WD, Brambilla E, Muller-Hermelink HK, et al. Pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon, France: IARC Press, 2004:145-247.
- Patel S, Macdonald OK, Nagda S, et al. Evaluation of the role of radiation therapy in the management of malignant thymoma. Int J Radiat Oncol Biol Phys 2012;82:1797-801. [Crossref] [PubMed]
- Ellison LF. Differences in cancer survival in Canada by sex. Health Rep 2016;27:19-27. [PubMed]
- Majek O, Gondos A, Jansen L, et al. Sex differences in colorectal cancer survival: population-based analysis of 164,996 colorectal cancer patients in Germany. PLoS One 2013;8:e68077. [Crossref] [PubMed]
- Bohanes P, Yang D, Chhibar RS, et al. Influence of sex on the survival of patients with esophageal cancer. J Clin Oncol 2012;30:2265-72. [Crossref] [PubMed]
- Jonklaas J, Nogueras-Gonzalez G, Munsell M, et al. The impact of age and gender on papillary thyroid cancer survival. J Clin Endocrinol Metab 2012;97:E878-87. [Crossref] [PubMed]
- Cardillo G, Carleo F, Giunti R, et al. Predictors of survival in patients with locally advanced thymoma and thymic carcinoma (Masaoka stages III and IVa). Eur J Cardiothorac Surg 2010;37:819-23. [Crossref] [PubMed]
- Ahmad U, Yao X, Detterbeck F, et al. Thymic carcinoma outcomes and prognosis: results of an international analysis. J Thorac Cardiovasc Surg 2015;149:95-100, 101.e1-2. Erratum in: J Thorac Cardiovasc Surg 2016;151:1220.
- Cardillo G, Rea F, Lucchi M, et al. Primary neuroendocrine tumors of the thymus: a multicenter experience of 35 patients. Ann Thorac Surg 2012;94:241-5; discussion 245-6. [Crossref] [PubMed]
- Ried M, Marx A, Götz A, et al. State of the art: diagnostic tools and innovative therapies for treatment of advanced thymoma and thymic carcinoma. Eur J Cardiothorac Surg 2016;49:1545-52. [Crossref] [PubMed]
- Ströbel P, Bauer A, Puppe B, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol 2004;22:1501-9. [Crossref] [PubMed]
- de Montpréville VT, Macchiarini P, Dulmet E. Thymic neuroendocrine carcinoma (carcinoid): a clinicopathologic study of fourteen cases. J Thorac Cardiovasc Surg 1996;111:134-41. [Crossref] [PubMed]
- Gaude GS, Hattiholi V, Malur PR, et al. Primary neuroendocrine carcinoma of the thymus. Niger Med J 2013;54:68-71. [Crossref] [PubMed]
- Bentz BG, Singh B, Woodruff J, et al. Head and neck soft tissue sarcomas: a multivariate analysis of outcomes. Ann Surg Oncol 2004;11:619-28. [Crossref] [PubMed]
- Yu JB, Smith BD. NCI SEER public-use data: applications and limitations in oncology research. OncoTherapy Network, March 18, 2009. Available online: http://www.oncotherapynetwork.com
- Cunningham D, Allum WH, Stenning SP, et al. Preioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant Chemotherapy Compared With Surgery Alone for Locally Advanced Cancer of the Stomach and Cardia: European Organisation for Research and Treatment of Cancer Randomized Trial 40954. J Clin Oncol 2010;28:5210-8. [Crossref] [PubMed]
- Savic M, Kontic M, Ercegovac M, et al. Comparison of mediastinal lymph node status and relapse pattern in clinical stage IIIA non‐small cell lung cancer patients treated with neoadjuvant chemotherapy versus upfront surgery: A single center experience. Thorac Cancer 2017;8:393-401. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007;357:1810-20. [Crossref] [PubMed]
- Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 2013;14:1208-15. [Crossref] [PubMed]


