Different strategy of salvage esophagectomy between residual and recurrent esophageal cancer after definitive chemoradiotherapy
Introduction
Chemoradiotherapy (CRT) is one of the treatment modalities for esophageal squamous cell carcinoma (ESCC) (1), and is indicated in most disease stages, from potentially resectable to metastatic disease. The evidence for CRT in this disease has been mixed, with previous studies demonstrating effectiveness (2,3), while others have reported local failure rates ranging from 45% to 66% (4-6). Patients who experience treatment failure with CRT may either present with a recurrence of ESCC following treatment completion, or with the histological identification of residual disease following treatment. For both groups, salvage esophagectomy offers the only chance of long-term survival (7,8). Owing to the significant associated risks (9-13) as well as its uncertain curative potential (14), many surgeons are hesitant to perform salvage esophagectomy. If it is possible to identify a subset of patients with ESCC who are likely to benefit from this risky procedure, more effective patient profiling for surgical candidates will be possible.
A previous study suggested that, following salvage esophagectomy, patients with residual cancer appear to have worse survival outcomes in than patients with cancer recurrence (15,16). Information regarding the survival benefits of salvage esophagectomy is required for identification of the clinical factors most likely to cause mortality in patients with residual cancer and in those with cancer recurrence. However, no previously published studies have discussed differences in strategies for the two types of patients. Therefore, in this study, we analyzed patients who underwent salvage esophagectomy to identify clinical limiting factors for survival, in both residual and recurrent ESCC.
Methods
Patients
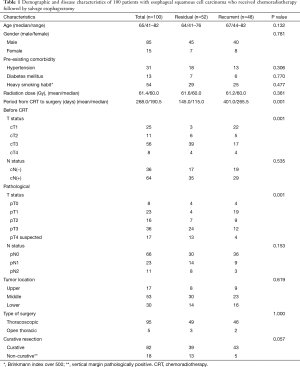
The present retrospective study protocol was reviewed and approved by the ethical committee of the Tohoku University School of Medicine (No. 2017-1-457). Between December 2001 and January 2016, 114 patients with esophageal cancer were treated with definitive CRT, followed by a salvage esophagectomy at the Tohoku University Hospital, Japan. From these cases, we selected cases using the following criteria: (I) the tumor was located at the thoracic esophagus; (II) subtotal esophagectomy was performed, excluding esophageal stripping; (III) residual or recurring ESCC was proved before salvage surgery; (IV) all records of pre-operative parameters were available. Patients who received neoadjuvant CRT were excluded. Fourteen of the identified medical records did not meet the eligibility criteria, resulting in a final sample of 100 patients. Demographics and disease characteristics are presented in Table 1.
Full table
Treatment
Endoscopy, computed tomography (CT) and/or positron emission tomography (PET) were used to determine initial staging. At the point of initial treatment, patients with stage I disease generally underwent surgery and patients with stage II/III usually received neoadjuvant chemotherapy followed by surgery. Patients who had potentially resectable cancer but wished to receive CRT could undergo definitive CRT for curative treatment (1-6). In addition, definitive CRT was also adapted for patients whose medical condition was unfitted. All patients undergoing CRT received continuous 5-fluorouracil (5-FU) and cisplatin, with concomitant radiotherapy either at our institution or at a related hospital. In most cases, the CRT schedule followed the protocol previously described by our institution (4). The initial target volume included the primary tumor and metastatic lymph node as well as the supraclavicular, mediastinal, and celiac lymph nodes in all patients. The irradiation dose was generally 60 Gy, ranging from 50.4 to 70 Gy, depending on the institution and patient condition. In addition, three patients received radiation dose of less than 60 Gy and 23 patients received larger dose. The effectiveness of the CRT was evaluated at least 1 month after the last radiation therapy. A complete response was defined as no evidence of residual cancer visible on CT scan and endoscopic biopsy.
Follow-up after definitive CRT was performed with triannual CT scan and triannual endoscopic exam including biopsy. PET scan was also used in cases of suspected recurrence. As definitive CRT is not neoadjuvant therapy, surgical resection would not be planned prior to the pathological demonstration of cancer cells. Treatment with salvage esophagectomy was considered for patients who had residual tumor postoperatively or patients with confirmed recurrence of ESCC. Pathological diagnosis of malignant cells by means of endoscopic biopsy was a necessary indication for salvage surgery. In the present study population, salvage esophagectomy would be planned shortly after diagnosis if the patient was in agreement. However, not all patients with recurrent or persistent cancer can receive salvage surgery; distant metastasis or significant lymph node metastasis are exclusion criteria for salvage surgery. Patients who were medically unfitted for esophagectomy before CRT would not be candidates for salvage surgery. In addition, severe coexisting condition such as decreased respiratory function, cardiac failure or poor performance status (2 and more) were exclusion for this surgery, which are same exclusion criteria we use for usual esophagectomy. All esophagectomy cases were performed at our hospital, most by thoracoscopy, and the remainder with an open thoracic procedure. The extent of lymph node dissection has been gradually reduced in salvage esophagectomy as a means to minimize risk (10). Lymph nodes selected for lymphadenectomy included lymph nodes located in the paraesophageal area and any lymph node suspected to be metastatic. The bronchial artery was preserved, provided no evidence of tumor invasion or inflammation was observed. In all cases, an anastomosis between the reconstructed organ and the cervical esophagus was performed at the left side of the cervix. There were no changes in these surgical practices over the study period, with the exception of the extent of lymph node dissection.
All specimens were examined at the Tohoku University Department of Pathology. The TNM classification (Union for International Cancer Control, 7th version) was used, and patients were staged pathologically. “Pathological T3” did not include cases in which the vertical margin (circumferential resection margin) was positive because those cases could have deeper cancer invasion than the adventitia. For this reason, “Pathological T4 suspected” was defined as the invading cancer cells were observed at the resected adjacent organ or at the vertical margin of the surgical specimen. No cases had positive horizontal margins (longitudinal resection margin). All patients underwent regular follow-up at intervals of 4 to 6 months for the first 5 years postoperatively. A CT scan and upper endoscopy were performed at every follow-up visit to rule out the possibility of recurrence.
Definitions
The indication for surgery is either “residual tumor” or “recurring tumor”. A “residual tumor” is a tumor that has persisted with no diagnosis of complete response ever made by CT and endoscopic exam. “Recurrence of tumor” was defined as a condition in which the tumor had been in complete response for at least 3 month after CRT, proven by CT and precise endoscopic examination. Endoscopic biopsy was performed in cases in which even a small suspicious lesion had been detected.
“Post-operative complications” were defined according to the Esophageal Complications Consensus Group (ECCG) definitions (17). In detail, “recurrent laryngeal nerve palsy” was defined as “any dysfunction in the vocal cords assessed by laryngoscopy on the next day after surgery”. “Anastomosis leakage” was defined as full thickness gastrointestinal (GI) defect involving anastomosis, staple line or conduit, confirmed by endoscopy or by contrast radiography in all cases. “Postoperative pneumonia” was determined as follows: an infiltration shadow seen on chest radiography, a demonstrated increase in inflammation, and the administration of antibiotics for pneumonia. “Post-operative hemorrhage” implied that “another operation was required for hemostasis”. “Death by other reason” refers to cases in which patients were discharged to home after surgery but died during follow-up for the reasons other than cancer.
Statistics
The Wilcoxon rank-sum test was used for intergroup comparisons of all continuous variables, whereas Fisher’s exact two-tailed test and Pearson’s test were used to compare categorical data. Overall survival (OS) estimates were determined using the Kaplan-Meier methods, and included all causes of death. Survival time was calculated from the time of salvage surgery to any cause of death. The statistical significance of the survival differences was determined using the log rank test. Patients who lived for more than 5 years were right censored. The Cox proportional hazards model was also used for univariate OS analyses. These analyses were performed electronically with JMP Pro 11.0.0 statistical software (SAS, Cary, NC, USA). A value of P<0.05 was considered significant.
Results
Patient demographics and disease burden are summarized in Table 1. The doses of radiation which administered prior to salvage surgery were similar between the two groups. Among the patients with residual cancer, salvage esophagectomy was performed an average of 145.0 days after CRT, whereas the average was 401.0 days among the patients with cancer recurrence. Tumors in patients with residual cancer tended to be more advanced than those of the patients with recurrent cancer in both the pre-treatment and pathological period. Nodal status of the TNM classification showed the same trend but was not statistically significant. Among five cases of open chest esophagectomy, two cases were converted from thoracoscopic surgery because of strong adhesion to the adjacent organ. Among 18 cases of non-curative resection, the vertical margin was pathologically positive for cancer cells in 17 cases and cancer cells were demonstrated in the pleural membrane in one case, which was regarded as a failure of curative resection. In addition, 25% of the patients with residual type cancer and 10% of patients with recurrent type cancer could not undergo curative resection.
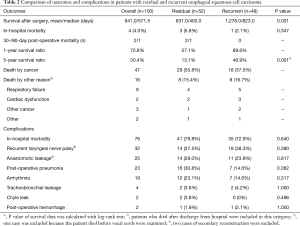
Approximately half of the study population died from the cancer as shown in Table 2. The rates of in-hospital mortality and morbidity were almost the same between the two groups. Although the table appears to suggest that patients with residual cancer have worse survival, their baseline is different. Recurrent laryngeal nerve palsy was observed in 32 patients proven by laryngoscopy; most experienced temporary palsy, but five cases lasted more than 1 year after surgery. Post-operative pneumonia was twice as frequent in patients with residual cancer as in patients with recurrent cancer. Most cases of arrhythmia were paroxysmal atrial fibrillation.
Full table
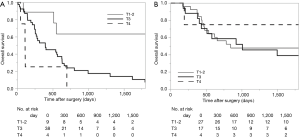
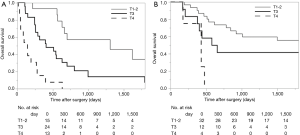
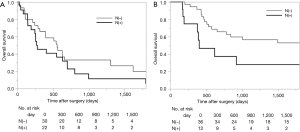
Survival analysis in terms of preoperative factors showed that T status prior to CRT correlated with survival in the group with residual ESCC (Figure 1A; P=0.010), but this correlation was not observed in the group with recurrent ESCC (Figure 1B; P=0.635). Conversely, pathological T status showed a correlation with survival in patients with both residual (Figure 2A; P<0.001) and recurrent cancer (Figure 2B; P=0.001). Among patients with pathological T3 cancer, the group with recurrent ESCC showed better survival, similar to T0-2 patients, while worse survival was demonstrated in the group with residual ESCC. N status before CRT (P=0.082) and pathological N status (Figure 3A; P=0.212) appear to have some relation to survival in patients with residual ESCC; however, these differences did not achieve significance. In the group with recurrent ESCC, N status prior to CRT did not correlate with survival (P=0.895); however, pathological N status appears to have some correlation with prognosis (Figure 3B; P=0.033).
Discussion
Salvage esophagectomy is the only potential curative option for patients with recurrent or residual ESCC after definitive CRT (7,8). However, the decision to perform salvage esophagectomy in these patients is difficult because this surgery is often risky, as documented in previous reports (9-15), which are consistent with our morbidity data (Table 2). Because of the effects of the radiation administered to the mediastinum and the tissues used as conduits, it is natural that the morbidity rate of patients undergoing this surgery would increase. It is necessary to create a novel surgical method or strategy to avoid such risks; at the same time, it is also important to identify those patients who would and would not derive the benefit from this treatment.
Salvage esophagectomy appears to achieve different clinical outcomes in patients with residual cancer and those with recurrent cancer. Previous reports have suggested that non-residual cancer is an important factor involved in long-term survival, as well as early T factor and R0 resection (15). A large multicenter study using propensity score also demonstrated that residual cancer appears to have poorer survival in general (16), a finding compatible with our results. This fact seems logical because cancer cells could survive not only in the main tumor but also in location other than the surgical site, which can lead to recurrence after salvage surgery. However, as far as we know, precise assessment of long-term survival in patients with residual and non-residual has not been performed in any previous study. To establish a strategy and treatment algorithm for those patients, classification by residual and recurrence disease is necessary because these cancer types usually follow different clinical course.
According to our data, the group of patients with recurrent ESCC had early stage cancer compared with the group with residual ESCC (Table 1), a tendency also observed in a previous report (16). This phenomenon occurs because patients undergoing CRT required close endoscopic follow-up, which leads to the discovery of cancer recurrence earlier than that of residual cancer already persist at the original site. Accordingly, there should be a difference in survival between these two groups, as shown in Table 2. Interestingly, although statistical significance was not achieved, the residual ESCC group had frequent complications, especially post-operative pneumonia. One explanation for this finding could be that strength and nutrition were insufficiently restored in those patients with residual cancer who underwent salvage esophagectomy just a few months after the completion of CRT. To avoid those complications, supportive nutrition and rehabilitation during and after CRT might be useful (18,19).
In the group of patients with residual ESCC, the important prognostic factor for salvage surgery was both pre-treatment and pathological T factor. Moreover, the pretreatment survival curve for each T status seems to be similar to the pathological survival curve. Because these are residual cancer cases in which cancer somehow could persist at the layer where cancer had existed before CRT, it is not surprising that pretreatment T status is almost equal to pathological T status. Interestingly, patients with T3 disease showed significantly poor survival compared to those with recurrent disease. On the other hand, both the pretreatment and pathological lymph node status, which was reported as the most important prognostic factor in patients with esophageal cancer who underwent surgery without pre-operative therapy (20), showed little correlation with prognosis, as demonstrated in a previous report (15). These results suggest that local control treatment such as esophagectomy and lymphadenectomy might not be sufficient as radical treatment for advanced residual cases, because these cancers were resistant to CRT and might have high malignant potential. Above all, salvage esophagectomy for residual ESCC should be considered in cases in which the tumor does not invade the adventitial layer prior to CRT. Lymph node metastasis is not an important factor in determining the necessity of this surgery.
In a group of patients with recurrent ESCC, pretreatment T status did not affect survival. Because those cases of ESCC occur newly or regrow from tiny clumps of surviving cancer cells, it is natural that the recurrent cancer would not occupy the same layer in which they had existed before, and their survival would not relate to the previous cancer level. For the same reason, the pretreatment N status did not correlate with survival at all. Our data show that suspected pathological T4, in which non-curative surgery may be considered, and pathological lymph node metastasis were worse prognostic factors, which is similar to the usual outcome of ESCC (20). An important issue becomes how best to diagnose unresectable tumor invasion and lymph node metastasis prior to surgery. Ordinal CT scans may not distinguish post-inflammatory changes and fibrosis from cancer cells. Recently, there have been a few reports of endoscopic ultrasonography (EUS) performed in cases of neoadjuvant CRT or definitive CRT (21,22). Although it is insufficient to detect small cancer foci in the fibrotic tissue, EUS seems to be useful to determine whether CRT was effective or not. Because EUS can obtain precise images of tumors, this modality may detect invasion to adjacent organs on further evaluation. Additionally, the accuracy of pre-surgical diagnosis of lymph node metastasis has a 50% sensitivity rate and an 83% specificity rate by CT, even in cases without neoadjuvant treatment (23). Further studies are needed to improve the accuracy of determining lymph node metastasis after CRT as well as the depth of tumor invasion.
In conclusion, patients with residual cancer after CRT may benefit from salvage esophagectomy if the cancer has not invaded to the adventitia at the time of CRT and surgery. Lymph node metastasis is not a prognostic factor for patients with residual cancer. On the other hand, patients whose cancer recurred after complete response by CRT might benefit from salvage surgery if the cancer appears resectable. Both T and N status before CRT are not important factors in consideration of salvage esophagectomy in cases of recurrence.
Acknowledgements
We thank “Editage” for assistance in editing the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The present retrospective study protocol was reviewed and approved by the ethical committee of the Tohoku University School of Medicine (No. 2017-1-457).
References
- Daly JM, Fry WA, Little AG, et al. Esophageal cancer: results of an American College of Surgeons Patient Care evaluation study. J Am Coll Surg 2000;190:562-72. [Crossref] [PubMed]
- Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160-8. [Crossref] [PubMed]
- Teoh AY, Chiu PW, Yeung WK, et al. Long-term survival outcomes after definitive chemoradiation versus surgery in patients with resectable squamous carcinoma of the esophagus: results from a randomized controlled trial. Ann Oncol 2013;24:165-71. [Crossref] [PubMed]
- Ariga H, Nemoto K, Miyazaki S, et al. Prospective comparison of surgery alone and chemoradiotherapy with selective surgery in resectable squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys 2009;75:348-56. [Crossref] [PubMed]
- Adams R, Morgan M, Mukherjee S, et al. A prospective comparison of multidisciplinary treatment of esophageal cancer with curative intent in a UK cancer network. Eur J Surg Oncol 2007;33:307-13. [Crossref] [PubMed]
- Ohtsu A. Chemoradiotherapy for esophageal cancer: current status and perspectives. Int J Clin Oncol 2004;9:444-50. [Crossref] [PubMed]
- Gardner-Thorpe J, Hardwick RH, Dwerryhouse SJ. Salvage oesophagectomy after local failure of definitive chemoradiotherapy. Br J Surg 2007;94:1059-66. [Crossref] [PubMed]
- Kumagai K, Mariosa D, Tsai JA, et al. Systematic review and meta-analysis on the significance of salvage esophagectomy for persistent or recurrent esophageal squamous cell carcinoma after definitive chemoradiotherapy. Dis Esophagus 2016;29:734-9. [Crossref] [PubMed]
- Nishimura M, Daiko H, Yoshida J, et al. Salvage esophagectomy following definitive chemoradiotherapy. Gen Thorac Cardiovasc Surg 2007;55:461-4. [Crossref] [PubMed]
- Tachimori Y, Kanamori N, Uemura N, et al. Salvage esophagectomy after high-dose chemoradiotherapy for esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg 2009;137:49-54. [Crossref] [PubMed]
- Miyata H, Yamasaki M, Takiguchi S, et al. Salvage esophagectomy after definitive chemoradiotherapy for thoracic esophageal cancer. J Surg Oncol 2009;100:442-6. [Crossref] [PubMed]
- Takeuchi H, Saikawa Y, Oyama T, et al. Factors influencing the long-term survival in patients with esophageal cancer who underwent esophagectomy after chemoradiotherapy. World J Surg 2010;34:277-84. [Crossref] [PubMed]
- D'Journo XB, Michelet P, Dahan L, et al. Indications and outcome of salvage surgery for oesophageal cancer. Eur J Cardiothorac Surg 2008;33:1117-23. [Crossref] [PubMed]
- Chen Y, Lu Y, Wang Y, et al. Comparison of salvage chemoradiation versus salvage surgery for recurrent esophageal squamous cell carcinoma after definitive radiochemotherapy or radiotherapy alone. Dis Esophagus 2014;27:134-40. [Crossref] [PubMed]
- Watanabe M, Mine S, Nishida K, et al. Salvage esophagectomy after definitive chemoradiotherapy for patients with esophageal squamous cell carcinoma: Who Really Benefits from this High-Risk Surgery? Ann Surg Oncol 2015;22:4438-44. [Crossref] [PubMed]
- Markar S, Gronnier C, Duhamel A, et al. Salvage surgery after chemoradiotherapy in the management of esophageal cancer: Is it a viable therapeutic option? J Clin Oncol 2015;33:3866-73. [Crossref] [PubMed]
- Low DE, Alderson D, Cecconello I, et al. International consensus on standardization of data collection for complications associated with esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286-94. [Crossref] [PubMed]
- Inoue J, Ono R, Makiura D, et al. Prevention of postoperative pulmonary complications through intensive preoperative respiratory rehabilitation in patients with esophageal cancer. Dis Esophagus 2013;26:68-74. [Crossref] [PubMed]
- Ligthart-Melis GC, Weijs PJ, te Boveldt ND, et al. Dietician-delivered intensive nutritional support is associated with a decrease in severe postoperative complications after surgery in patients with esophageal cancer. Dis Esophagus 2013;26:587-93. [Crossref] [PubMed]
- Peyre CG, Hagen JA, DeMeester SR, et al. Predicting systemic disease in patients with esophageal cancer after esophagectomy: a multinational study on the significance of the number of involved lymph nodes. Ann Surg 2008;248:979-85. [Crossref] [PubMed]
- Owaki T, Matsumoto M, Okumura H, et al. Endoscopic ultrasonography is useful for monitoring the tumor response of neoadjuvant chemoradiation therapy in esophageal squamous cell carcinoma. Am J Surg 2012;203:191-7. [Crossref] [PubMed]
- Shim CN, Song MK, Lee HS, et al. Prediction of survival by tumor area on endosonography after definitive chemoradiotherapy for locally advanced squamous cell carcinoma of the esophagus. Digestion 2014;90:98-107. [Crossref] [PubMed]
- van Vliet EP, Heijenbrok-Kal MH, Hunink MG, et al. Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer 2008;98:547-57. [Crossref] [PubMed]


