Recent progress and market analysis of anticoagulant drugs
Introduction
Vascular thrombosis describes clot formation within blood vessels or cardiac chambers. Intravascular thrombosis can be induced by many factors, but the fundamental ones are excessive blood coagulation and platelet adhesion aggregation. It is associated with various diseases manifesting with ischemia and infarction caused by vascular lumen stenosis or occlusion. Thrombosis is related to cardiovascular morbidity and mortality (1).
Thrombosis is classified into arterial and venous thrombosis. Arterial thrombosis is typically initiated at sites of atherosclerotic lesions by activation of platelets on the arterial wall, eventually leading to clinical disease, such as acute myocardial infarction and stroke. Venous thrombosis is triggered by a variety of causes within the venous system, eventually leading to venous thromboembolism (VTE) (2). VTE is the third common cardiovascular disease after acute coronary syndrome and stroke (3).
Epidemiology
Thrombosis results in significant morbidity and mortality. The incidence of venous thrombosis is 3‰ in the general population. In the U.S., 100,000–300,000 people die from venous thrombosis every year, and the number of hospital admissions exceeds 500,000 per year. Further, the prevalence of venous thrombosis has been rising in recent years. In Europe, there are 500,000 deaths due to venous thrombosis every year, and the number is more than the sum of the deaths caused by AIDS, breast cancer, prostate cancer and traffic accidents (4). In the past, venous thrombosis was regarded as a rare disease in China, but its occurrence has escalated rapidly in the twenty-first century. The number of hospital admissions has increased by 10–30 times in recent years, predominantly due diagnosis and treatment of pulmonary embolism (PE). The overall incidence of venous thromboembolism is about 25.5–50% in orthopedic inpatients, 10–30% in patients admitted to the intensive care unit, and those with chronic obstructive pulmonary disease, lung cancer, and high age (5).
Market trends
Global market
IMS data showed that the global market sales of anti-thrombotic drugs were USD$23.5 billion in 2013, accounting for 53.1% of the sales of drugs for cardiovascular diseases and 2.7% of global drug sales. The global market of anti-thrombotic drugs is close to a saturation, with fierce competition. However, the global market of anti-thrombotic drugs has gradually expanded with market sales expected to reach USD$25.9 billion in 2018 (6).
Globally, anti-platelet drugs performed best, with global drug sales reaching $9.5 billion, accounting for 40.4% of all anti-thrombotic drugs sales. Clopidogrel (trade name: Plavix) is the leader of anti-platelet drugs produced by Sanofi/Bristol Myers Squibb Company. The global drug sales were USD$2.3 billion in 2013. However, clopidogrel has its limitations, such as not being able to be metabolized by those patients with CYP2C19 gene mutations, which has led theFDA to issued a black-box warning. After the drug patent of Plavix expired in May 2012, global sales decreased sharply. It has been projected that sales of anti-platelet aggregation drugs will decline to 19.3% in 2018, and the tis class will lose the leading position of anti-thrombotic drugs. With the appearance of new direct thrombin inhibitor and direct coagulation factor Xa inhibitors, anti-clotting drugs will face new challenges in future markets. The global drug sales of direct thrombin inhibitors were USD$2.4 billion in 2013, which is the third largest percentage of anti-thrombotic drugs, accounting for 10.4% of all anti-thrombotic drug sales. Dabigatran etexilate was leading sales statistics, accounting for 73.6% of similar drugs sales. The global sales of direct coagulation factor Xa inhibitors were USD$2.1 billion in 2013, accounting for 9.6% of all anti-thrombotic drugs sales. Rivaroxaban, produced by Bayer, accounted for 93.6% of similar drugs sales. The sales of direct coagulation factor Xa inhibitor drugs is expected to account for 32.6% of anti-thrombotic drugs sales in 2018 (6).
The global sales of anticoagulant drugs was equivalent to USD$13.413 billion and USD$12.761 billion in 2012 and 2013 respectively. The overall market is relatively stable. The sales of Clopidogrel declined rapidly due to its patent expiration, while the new Xa factor inhibitors, Rivaroxaban and Apixaban, increased rapidly (Table 1).
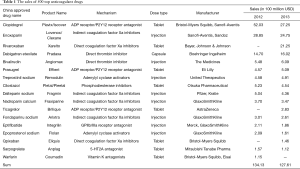
Full table
Domestic Chinese market
According to the IMS data, domestic Chinese market sales of anti-thrombotic drugs increased from $9.636 to $12.453 billion between 2012–2014. The anti-platelet drugs accounted for the largest proportion, but the market share of direct coagulation factor Xa and direct thrombin inhibitors increased relatively faster, with an average annual growth rate of more than 40% (Table 2).
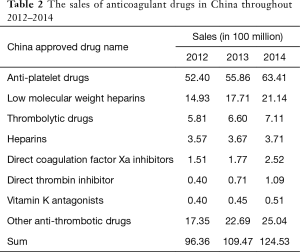
Full table
According to MENET data, the sales of anticoagulant drugs in public hospitals in Chinese cities were $12.287 and $11.522 billion in 2013 and 2014, respectively. The average annual growth rate was 12%. The market share of clopidogrel accounted for 53% of the overall anticoagulant drug sales market in 2014. From the perspective of market prospect, the average sales growth rates of rivaroxaban, argatroban, ticagrelor, bivalirudin, Dabigatran etexilate, apixaban and eptifibatide were more than 40% (Table 3).
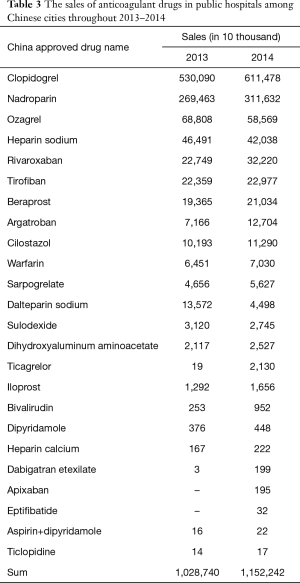
Full table
History of anti-coagulant drug
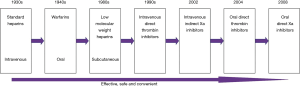
Since the first anti-coagulant heparin was introduced in the 1930s, the development of novel and improved anti-coagulant drugs has continued (7). Traditional anti-coagulants include heparin (UFH), low molecular weight heparin (LMWH) and warfarin. Their effects of preventing and treating thromboembolic disease have been confirmed by numerous clinical trials, and thus anticoagulant drugs have been widely used in medicine. However, these drugs have some obvious disadvantages. For example, their pharmacodynamics and pharmacokinetics are unpredictable, leading to risk of bleeding. Laboratory monitoring is thus necessary to adjust the dose (e.g., for heparin and warfarin). Thrombocytopenia and osteoporosis induced by heparin is associated with significant risk of complications. Besides, parenteral administration (e.g., heparin, low molecular weight heparin) is inconvenient in particular for patients discharged from the hospital. New oral anti-coagulant drugs that are orally administered, not affected by food or drug interactions, safe and effective with fixed dose and without the need to monitor are desired (8). Therefore, research and development of novel anticoagulant agents interacting with different coagulation factors in the coagulation cascade are emerging.
At present, new anticoagulant drugs include direct thrombin inhibitor, factor Xa inhibitor, factor IX inhibitor, tissue factor inhibitor and novel vitamin K antagonists. Among them, direct thrombin inhibitor (DTI) and factor Xa (FXa) inhibitors are most representative.
Representative drugs of direct thrombin (factor IIa) inhibitors include Hirudin, recombinant hirudin, bivalirudin, argatroban (withdrawn from foreign countries due to hepatotoxicity), dabigatran and etexilate. Representative drugs of selective factor Xa inhibitors include fondaparinux, biotinylated idraparinux, rivaroxaban, apixaban and edoxaban. The former two are indirect factor Xa inhibitors, whereas the latter 3 are direct factor Xa inhibitors (Figure 1).
Classification and clinical application of anticoagulant drugs
Anticoagulant drugs can be classified into anti-platelet drugs and anticoagulant agents. Anti-platelet drugs, or platelet aggregation inhibitors, inhibit platelet adhesion, aggregation and secretion through acting on ADP receptors, inhibiting the four arachidonic acid (AA) metabolism and increasing platelet cyclic adenosine monophosphate (cAMP) concentration. Anticoagulant agents prevent blood clotting, by removing or inhibiting certain coagulation factors in the blood. The traditional natural anticoagulant agents include heparin, low molecular weight heparin, hirudin, whereas novel anticoagulant agents include rivaroxaban and dabigatran etexilate.
Anti-platelet drugs
Arterial thrombosis caused by rupture or erosion of atherosclerotic plaques is the main cause of ACS. Therefore, multiple platelet signaling pathways mediating thrombosis are potential therapeutic targets. Currently, there are three types of anti-platelet drugs approved for clinical treatment of ACS or secondary prevention, including oral cyclooxygenase-1 (COX-1) inhibitors, GP II b/III a receptor inhibitors and oral P2Y12 receptor inhibitors (9-12).
Although the combination of aspirin and clopidogrel dual antiplatelet therapy can benefit patients, there is still a considerable number of patients who experienced adverse events due to individual differences caused by the anti-platelet effects of clopidogrel. Patients with platelet hyperresponsiveness after clopidogrel treatment have a corresponding increase in the risk of recurrent thrombosis, and even COX-1 and P2Y12 receptor are fully inhibited. Platelets can be activated by other pathways to induce thrombosis, which has led to the development of novel anti-platelet agent (13).
COX-1 inhibitor-aspirin
Aspirin irreversibly restrains COX-1, blocks the formation of thromboxane A2 (TXA2), and inhibits G protein-coupled TXA2 receptors and thromboxane prostaglandin receptor (TP) mediated platelet activation (14). A series of studies have shown that aspirin can significantly reduce the incidence of cardiovascular events in patients with ACS. The most beneficial dose is between 75–100 mg, and the benefits would not be increased with higher dose. However, the risk of bleeding in patients with aspirin treatment is increased in a dose-dependent manner, especially the upper digestive tract bleeding. Considering the aspirin restrain COX-1 specifically, it cannot effectively inhibit platelet activation and thrombosis caused by other factors, such as two adenosine monophosphate (ADP) and blood clotting enzyme (15).
GP IIb/IIIa receptor inhibitor
Platelet membrane glycoprotein GP IIb/IIIa receptor inhibitor can block the last pathway of platelet aggregation by competing GP IIb/III a receptor with fibrinogen and von Willebrand factor (vWF). At present, platelet membrane glycoprotein GP IIb/III a receptor inhibitors used in clinical application can be divided into three categories: Monoclonal antibodies such as abciximab; synthetic peptide inhibitors such as eptifibatide; non-peptide inhibitors such as tirofiban, xemilofiban, lefradafiban. Among them, the intravenous infusion of GP IIb/III A inhibitors (abciximab, eptifibatide, tirofiban) are fast and durable, and can reduce the ischemic events in patients with ACS undergoing PCI surgery and improve the prognosis. Therefore, intravenous injection of GP IIb/IIIa is a reasonable choice to patients with high risk ACS undergoing PCI, but with a higher incidence of bleeding complications. Although oral GP IIb/III a receptor inhibitors (xemilofiban, lefradafiban) can be applied to long term treatment, this use has not been approved due to the bleeding problem (16,17).
P2Y12 receptor inhibitor
Under the induction of ADP, G protein-coupled purine receptor P2Y12 can enhance and maintain the activation state of platelets. Dual-antiplatelet therapy (DAPT), which is based on a P2Y12 receptor inhibitor and aspirin, is a recommended regimen for acute and long-term ischemic events in patients with ACS (18). The addition of the P2Y12 inhibitor to aspirin can reduce platelet activity significantly compared to single agent. A series of clinical studies designed to study the best antithrombotic regimen in patients with coronary stenting have been evaluated to confirm this synergistic effect of DAPT. Notably, DAPT is targeted at TXA2 and ADP mediated platelet activation and has no effect on other pathways such as thrombin through PAR mediated platelet activation (19-22) (Table 4).

Full table
Ticlopidine and clopidogrel
Ticlopidine is the first FDA-approved P2Y12 receptor inhibitor and the first generation of thienopyridines. However, the incidence of side effects of the drug is high, including life-threatening blood abnormalities. Clopidogrel, as the second generation of thienopyridine drugs, is safer than ticlopidine. Currently, clopidogrel is the most widely used P2Y12 receptor inhibitor, and is the only one of these drugs recommended for stable coronary artery disease in patients undergoing PCI and thrombolytic therapy of acute ST-segment elevation myocardial infarction (STEMI) patients (23,24).
As noted previously, platelet hyperreactivity after clopidogrel treatment has led to recurrent thrombotic events in many patients after DAPT treatment, possibly due to higher heterogeneity among individuals. The heterogeneity may be derived from the clinical factors (malabsorption, drug reactions, ACS, diabetes, obesity, chronic kidney disease), genetic variants such as CYP polymorphism, and cytokines (accelerated platelet regeneration, decreased CYP3A4 metabolic activity or P2Y12 pathway upregulation).
Pharmacodynamic studies have confirmed that about 30–40% of patients receiving clopidogrel treatment remain having high platelet reactivity, leading to poor prognosis. Large-scale clinical trials found that increasing the dose of clopidogrel in patients would not be more beneficial (25).
Prasugrel
Prasugrel is the third generation thienopyridine, which is an oral prodrug that requires the production of active metabolites via the oxidation process including the hepatic CYP enzyme to take effect. However, metabolic transformation efficiency of prasugrel is higher, resulting in more effective in vivo. Compared with clopidogrel, the above pharmacological properties make prasugrel more rapid, more potent antiplatelet action and smaller inter-individual response variability (26).
The TRITON-TIMI38 trial has shown that prasugrel is superior in reducing the formation of arterial thrombosis in patients with PCI (27). In July 2009, the US FDA approved the drug for acute myocardial infarction and unstable angina patients undergoing PCI treatment. Although prasugrel can reduce the risk of thrombosis in patients after PCI, but it will increase the risk of bleeding. In addition, the platelet inhibition of prasugrel is irreversible.
Ticagrelor
Different from clopidogrel and prasugrel, ticagrelor is not a thienopyridine but a cyclopentatriazole pyridine. It is a non-prodrug without metabolic activation. Prasugrel can reversibly act on the ADP receptor subtype P2Y12, and significantly inhibit the platelet aggregation caused by ADP. Also, it takes effect more rapidly by oral administration. Experimental studies have shown that ticagrelor is more rapid, stronger and more homogeneous than clopidogrel in inhibiting platelets, and the combination of receptor is reversible (28).
Ticagrelor has been approved for the treatment and secondary prevention of all types of ACS patients, regardless of whether these patients received interventional therapy. And ticagrelor may be administered before coronary angiography evaluation, but also can be used for clopidogrel pretreatment in patients. However, ticagrelor has not yet gone through any tests relating to its effectiveness and safety in patients with stable coronary artery disease, so it is not yet approved for use among these patients (29,30).
Cangrelor
Although the clinical efficacy of prasugrel and tigriol is better than clopidogrel, both of them are orally administered with several clinical application limitations, such as heterogeneity of patients with drug response, slow loss in efficacy, dependence on oral administration, which limit its hemodynamic instability, taking sedatives, tracheal intubation, shock, hypothermia treatment or associated with nausea and vomiting in patients. Cangrelor is an intravenous injection ATP analogue that works by reversibly binding to P2Y12 receptors without metabolic transformation. Its inhibition of platelet is strong (>80%) and it can reach the steady-state concentration of blood after intravenous injection for a few minutes (31,32).
On June 22, 2015, cangrelor was approved by the FDA to prevent coronary artery blockage during PCI in adult patients in order to reduce perioperative myocardial infarction. Clinical trials comparing cangrelor with clopidogrel in more than 10,000 patients have shown that cangrelor significantly reduced heart attack, the occurrence of revascularization and stent thrombosis. The overall incidence of severe bleeding was low, but the cangrelor group was higher than the clopidogrel group (33).
PAR-1 receptor inhibitor
Thrombin is one of the strongest platelet activators, thrombin-mediated aggregation of platelet activation plays an important role in thrombosis. The response of platelets to thrombin is mediated by G protein-coupled receptors on the surface of platelets. There are four sub-types of human PAR widely distributed in tissues (34). PAR-1, PAR-3, PAR-4 can be activated by thrombin, whereas PAR-2 is activated by trypsin or tryptase without activation by thrombin. Human platelets express PAR-1 and PAR-4 receptor. Thrombin-mediated platelet activation is mediated by PAR-1 and PAR-4, while PAR-1 plays the most important role.
PAR-1 receptor antagonist is one of the most concerned and promising novel anti-platelet drugs. ADP and TXA2 platelet activation pathway is a critical step for routine pathological thrombosis and hemostasis. Therefore, the combination of cyclooxygenase inhibitor and ticlopidines plays a role in anti-embolism while inevitably leading to bleeding complications. In contrast, PAR-1 receptor-mediated platelet activation plays a role in pathologic thrombosis and is not necessary for protective hemostasis. Thus, PAR-1 receptor antagonist works on anti-thrombotic without increasing the risk of bleeding, so it is potentially an ideal anti-platelet drug (13). There are 2 main oral PAR-1 receptor antagonists: vorapaxar and atopaxar.
Vorapaxar
Vorapaxar inhibits TRAP-induced platelet aggregation by up to 80% within one week of administration. Since the plasma half-life of vorapaxar was 165–311 hours, TRAP-induced platelet aggregation was reduced by 50% in plasma at 4 weeks after drug withdrawal (13). Vorapaxar can reduce the incidence of cardiovascular events in patients with coronary heart disease, so in 2014, the FDA approved the use of vorapaxar in patients with recurrent myocardial infarction or peripheral vascular disease in order to reduce recurrent myocardial infarction, stroke, and sudden death. The result of TRA2P-TIMI-50 indicates that vorapaxar can increase the risk of intracranial haemorrhage in patients with a previous history of stroke, and therefore vorapaxar sulfate is contraindicated in patients with stroke, TIA, and intracerebral hemorrhage (35).
Atopaxar is novel reversible PAR-1 receptor antagonist. Its antiplatelet mechanism can selectively interfere thrombin-mediated platelet activation without increasing the risk of bleeding. The results of test J-LANCELOT, LANCELOT-ACS and LANCELOT-CAD showed that atopaxar was safe in patients with ACS or high-risk coronary artery disease, which reduced the incidence of adverse ischemic events, however, there was no statistical significance. The above-mentioned tests consistently showed that the use of atopaxar corrected QT interval prolongation and abnormal liver function. The phase III clinical trial is underway (36-38).
Thromboxane receptor antagonist
Although thromboxane A2 is the main agonist of the platelet activation pathway mediated by thromboxane receptors, other eicosanoids, such as isoprostaglandin and endoperoxide, are also important agonists of TP receptors that do not need COX enzyme. Although the treatment of aspirin blocks the formation of thromboxane A2, ischemic events still occur in patients.
Some researchers believe that TP receptor antagonists can bring more benefits than COX-1 inhibitors, and many TP antagonists also inhibit the synthesis of thromboxane A2. Currently, TP receptor antagonists including Daltroban, Sulotroban and Terutroban, thromboxane A2 synthase and TP receptor dual inhibitors including EV-077, Picotamide and Ridogrel. Yet, the clinical test results of TP receptor antagonists are disappointing. The benefits of Ridogrel which is used in patients with acute myocardial infarction are not better than aspirin; Terutroban and Picotamide have not proved to be superior to aspirin. It is noteworthy that EV-077 in healthy volunteers and patients with diabetes showed strong platelet inhibition, but it is still in phase II development. There is no progress of the drug-related clinical trials (39).
5-HT2 receptor inhibitor
5-HT
5-HT or Serotonin is widely distributed in the central nervous system and surrounding tissues as a neurotransmitter and vasoactive substance. The type of 5-HT receptor is complex. It has been found that there are at least seven categories in human so far (40). Because of the presence of a large number of 5-HT2 receptors on the vessel wall, its activation can significantly affect the integrity and secretion of vascular endothelial cells, the contractile function of vascular smooth muscle cells (SMC) and the development of atherosclerosis. Therefore, studies on the effects of 5-HT2 receptors on blood vessels are of great importance in the prevention and treatment of cardiovascular diseases (41).
Sarpogrelate
Sarpogrelate (SARP) is a 5-HT2 receptor inhibitor produced by Mitsubishi Pharmaceutical Co., Ltd. of Japan, which specifically binds to the 5-HT2 receptor. SARP is applied to cure peripheral vascular disease, such as chronic ischemic vascular occlusion, coronary heart disease, nervous system diseases, and thrombotic diseases. The pharmacological effects of SARP include the following aspects: inhibition of platelet aggregation, inhibition of 5-HT and platelet aggregation caused by vasoconstriction, antithrombotic function, improving collateral circulation and so on.
Studies have shown that Sarpogrelate reduces platelet aggregation and plasminogen activator inhibitor activity in patients with stable angina pectoris in the original basis of aspirin. This suggests a possible adjunctive effect of Sarpogrelate on patients with stable angina pectoris, which indicated a new direction for the wide application of Sarpogrelate (42).
Phosphodiesterase inhibitor
Phosphodiesterase inhibitor inhibits platelet agglutination by inhibiting phosphodiesterase activity. The representing drugs include dipyridamole, and cilostazol.
Dipyridamole
Dipyridamole was first used in clinic due to its role in vasodilator. Subsequent in vitro experiments found that dipyridamole can inhibit platelet aggregation, and it is therefore increasingly used as anti-platelet drugs. Its mechanism is inhibition of platelet adhesion to the vessel wall injury site, activation of adenylate cyclase to increase platelet cAMP synthesis, inhibition of PDE to reduce the decomposition of cAMP, inhibition of TXA2 formation, and increasing prostacyclin synthesis and activity (43). Dipyridamole has the disadvantage of poor chemical stability and short half-life. Double the dose course of sustained-release agents is necessary in order to maintain the role of anti-platelet activity. Clinical use of low-dose aspirin dipyridamole compound sustained-release formulations for the prevention of stroke are common.
Cilostazol
Cilostazol is a selective PDE III inhibitor that inhibits platelet aggregation and relaxes blood vessels directly and anti-inflammatory response, therefore preventing early deterioration of neurological function. The CSPS trial, an earlier study of cilostazol in the prevention of stroke, showed a 41.7% reduction in stroke risk (P=0.015) in the cilostazol group compared with the placebo group. And a 43.4% reduction in stroke risk (P=0.0373) in patients with lacunar infarction, which suggested that cilostazol has a specific effect on small vessel disease (44). CSPS-2 studies have shown that cilostazol may be superior to aspirin in the prevention of secondary stroke, with a lower incidence of bleeding events, which can be used for stroke prevention in noncardiogenic stroke patients (45).
Other anti-platelet drugs
Other new drugs that target multiple targets in the early phase of clinical progression to platelet aggregation signaling pathways include P2Y12 receptor antagonist MRS2179 (46), MRS2500 (47), oral GP IIb/IIIa inhibitors MNS, RUC-1 (48), platelet collagen receptor inhibitors such as GP VI antagonist Kistomin (49), Revacept (50), GP Ib antagonist 6B4-Fab monoclonal antibody (51), nitric oxide donor LA846,LA419 (52), prostaglandin E receptor 3 antagonist DG-041 (53), serotonin receptor inhibitor APD791 (54), and phosphatidylinositol 3-kinase inhibitor TGX-221 (55). Whether these drugs can be used in clinical practice would require further clinical trials.
Anticoagulant
Blood clotting is a complex chain reaction of proteolytic activation (56), which ultimately turns soluble fibrinogen into a stable, insoluble fibrin which clot with blood platelets. The complex system of blood coagulation, anticoagulation, and fibrinolysis system, ensures that blood fluidity without thrombosis or excessive bleeding. When the body is in a hypercoagulable state or anticoagulation and fibrinolysis is weakened, thromboembolic disease will occur.
Anticoagulants prevent or inhibit the clotting process by affecting one or more clotting factors in the blood, and are therefore useful for prevention and treatment of endovascular embolization or thrombosis, and prevention of stroke or other thrombotic diseases. Current anticoagulants include heparins, synthetic pentose, vitamin K inhibitors (VKAs), direct thrombin inhibitors and direct coagulation factor Xa inhibitors.
Heparins
Heparin is a glycosaminoglycan composed of glucosamine, L-iduronic acid, N-acetylglucosamine, D-glucuronic acid and their sulfated derivatives (57).
According to the molecular weight distribution, heparin can be divided into standard heparin and low molecular heparin. The molecular weight of standard heparin, also known as unfractionated heparin or unfractionated heparin, is 5–30 KD. Its average molecular weight is 12 KD. Low molecular heparin is unfractionated heparin by physical separation or chemical degradation of small molecules. Its molecular weight is about 3–8 KD, and the average molecular weight is 5 KD.
Standard heparin
Standard heparin is primarily used for anticoagulant and anti-thrombotic treatment of diffuse intravascular coagulation and anti-thrombosis caused by various causes (58), as well as in anti-coagulation during hemodialysis, cardiopulmonary bypass, catheterization, and micro-vascular surgery. Clinical application and research show that standard heparin also has a variety of other biological activity and clinical use, including anti-inflammatory, anti-allergic, hypolipidemic, anti-atherosclerosis, anti-medial SMC anti-cancer and so on. Yet, standard heparin needs continuous monitoring due to its adverse reactions including severe bleeding, thrombosis, and osteoporosis, and it cannot be orally administered.
Although low molecular heparin preparations have many advantages, such as strong curative effect and low side-effect, it is still challenging to replace standard heparin preparations in many clinical scenario including blood preservation, cardiac surgery, renal dialysis, and anti-arterial thrombosis. Further standard heparin has more beneficial therapeutic effects on chilblain, varicose veins, neurodermatitis, and superficial phlebitis. Standard heparin is safer for both pregnant and lactating women and their babies. For these reasons, the standard heparin formulations are still widely used clinically.
Low molecular heparin
In order to reduce the side effects of heparin, such as easy bleeding, thrombocytopenia and osteoporosis, Europe first developed low molecular heparin in late 1980s. Low molecular heparin and standard heparin are different in molecular weight and pharmacological effects. The ratio of anti-F Xa to anti-F IIa activity of standard heparin is usually about one. Low molecular weight heparin anti-coagulation factor Xa activity is increased, with anactivity greater than 70 U/mg. Anticoagulant factor IIa activity is decreased, with a resulting low molecular weight heparin anti-F Xa and anti-F IIa activity ratio of more than 1.5. Therefore, low molecular heparin separates the effect of anti-thrombotic and bleeding with a strong anti-thrombotic effect, while bleeding side effects are small (59).
Differences of low molecular heparin are secondary to different depolymerization methods, leading to different pharmacokinetic and anticoagulant activities. Therefore different low molecular weight heparin formulations cannot be substituted by each other in clinic. At present the international community has developed a dozen low molecular heparin products, such as Enoxaparin sodium, Nadroparin calcium, Dalteparin sodium, Parnaparin sodium, Tinzaparin sodium, and Reviparin sodium. Although low molecular heparin is safe, it cannot be orally administered and has no special antidote.
Synthetic pentosan sulfate
Fondaparinux is a synthetic pentosan sulfate and an indirect inhibitor of the antithrombin-dependent factor Xa. When fondaparinux selectively binds to antithrombin, the conformation of antithrombin changes, increasing the rate of antithrombin inhibitor Xa, and blocking the formation of thrombin by inhibiting factor Xa. Antithrombin play an inhibitory factor Xa role, fondaparinux that is antithrombin dissociation, but also with other antithrombin molecules (60).
Table 5 compares heparin and synthetic pentosan to prevent contact thrombus. The ability of general heparin to inhibit IIa is strong, followed by tinzaparin, dalteparin, nadroparin, enoxaparin and (fondaparinux). The ability of inhibiting contact thrombosis is weakened in turn from top to bottom. Factor IIa in the role of thrombosis cannot be ignored. The ratio of dalteparin anti-Xa and anti-IIa was more reasonable, which is safe for ACS patients receiving PCI treatment.
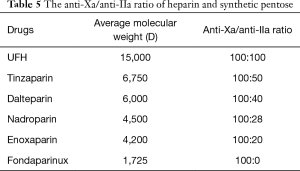
Full table
VKAs
VKAs inhibit the synthesis of prothrombin and factors VII, IX and X by antagonizing vitamin K. Because there is a storage of coagulation factors in the body, the effect of Vitamin Ksets in only after already synthesized and stored factors are exhausted. Anticoagulant effect, so its role began to slow, but the role of a longer duration, for longer time to be anticoagulants such as deep vein thrombosis and PE, when the amount of bleeding caused by improper use, in addition to vitamin K, the most important thing is to lose fresh blood to supplement the clotting factor.
Over the past 60 years, warfarin played an important role in the prevention and treatment of thromboembolic diseases as the most widely used oral VKAs (61). Although the anti-thrombotic effect of warfarin is clear, its metabolism is susceptible to food, drugs and other factors. Its treatment window is narrow, and needs frequent monitoring of patients coagulation dose titration to maximize the balance of anti-coagulation effect and bleeding risk, thus resulting in poor compliance of patients medication.
Direct thrombin inhibitors
Hirudin and lepirudin
Lepirudin was first developed by the German company Bayer, which was approved by the European Union to list in Germany in 1997, and was approved by the FDA to list in the United States in 1998. Currently it has been registered in more than 10 countries including Europe, the United States, Australia, New Zealand, South Africa etc. The structure of 63 of the tyrosine residue is not sulfated, and thus the Lepirudin anticoagulant is lower than natural hirudin, but significantly reduced bleeding adverse reactions. It is widely used in ACS and myocardial infarction, PCI and restenosis after coronary angioplasty (PTCA), heparin-induced thrombocytopenia, prevention of deep vein thrombosis and other aspects (62).
Bivalirudin
Bivalirudin is a polypeptide consisting of 20 amino acids with a molecular weight of 2,180, which has a direct inhibition of thrombin activity. Bivalirudin is capable of reversibly binding to fibrin-bound thrombin and circulating free thrombin with a short half-life, so bivalirudin does not present with ischemic and hemorrhagic complications that are often present with other existing anticoagulants. It was developed by American Medicines Company and was approved by American FDA in 2000. The trade name is Angiomax (63).
Argatroban
Argatroban is an anti-thrombotic drug first developed by the Japanese Mitsubishi Institute of Chemistry. The trade name is Novotel Sotec. As the world’s first selective antithrombin agent, argatroban was listed in Japan in February 1990. Argatroban, produced by Pfizer, was approved by the US FDA in 2000 and FDA-approved indications for the prevention or treatment of heparin-induced thrombocytopenia thrombosis in 2000, and approved for patients with high-risk percutaneous coronary intervention (PCI) with selective HIT or HIT in 2002. Although argatroban is highly active and has no effect on platelet function at therapeutic doses, it is more potent than hirudin and heparin in its antithrombotic effects, particularly fibrinogen-associated thrombi. There is no antigenic novel anticoagulant, but because of its narrow therapeutic window and lack of advantages compared to similar competing products, its sales is not as competitive as that of bivalirudin (64).
Dabigatran etexilate
Dabigatran etexilate was developed by Boehringer Ingelheim, Germany with a trade name Pradaxa. It was first listed in Germany and the United Kingdom in April 2008, which is the first novel category of oral anticoagulant drugs following the warfarin after 50 years. The listing of this product is a major advance in the field of anticoagulant therapy and potentially lethal thromboprophylaxis.
Dabigatran etexilate is turned into active dabigatran in vivo, which antagonizes both free and thrombus-bound thrombin. The peak concentration of plasma was 1.5 h after oral administration. The half-life was from 14 to 17 h, and the bioavailability was about 7.2%. It is mainly metabolized by kidney (65).
A clinical trial of RELY (66) aiming at the prevention of atrial fibrillation (AF) strokes in 18,000 patients showed that dabigatran etexilate 110 mg (bid) was comparable to warfarin in preventing stroke and systemic thrombosis, but the risk of major bleeding event was significantly reduced. Therefore, in 2011, “atrial fibrillation treatment guidelines” of the United States recommended that dabigatran etexilate can be used as warfarin replacement therapy. The pivotal data of RELY-ABLE trial further confirmed the long-term efficacy and safety of Pradaxa in stroke prevention in patients with nonvalvular AF.
Therefore, the FDA updated prescription information clarified that Pradaxa 150 mg is superior to warfarin in reducing both ischemic stroke and hemorrhagic stroke. At the same time, dabigatran etexilate was approved as the second long-term oral anticoagulant after warfarin.
Direct coagulation factor Xa inhibitor
Rivaroxaban
Rivaroxaban was developed by Bayer/Johnson with the trade name Xarelto. It was listed in Canada on September 15, 2008, in the European Union on October 1, 2008, and further listed in Australia in 2009. US FDA approval date is July 1, 2011.
Canada and the European Union approved the use of rivaroxaban for the prevention of venous thrombosis in patients undergoing elective total hip arthroplasty and total knee arthroplasty. After that, European regulators approved it for the treatment of DVT and the prevention of recurrent DVT and PE. In the United States, Rivaroxaban is used to prevent stroke in patients with nonvalvular AF, to treat deep vein thrombosis and PE, to reduce the risk of relapse, and to reduce the risk of deep vein thrombosis and PE after knee and hip replacement surgery.
Rivaroxaban belongs to the direct FXa inhibitor, which is the first direct oral small molecule FXa inhibitor. It has an inhibitory effect on both free and clot-bound FXa, and thus has a high anticoagulant property. The combination of Rivaroxaban with FXa is reversible and therefore it has a low incidence of bleeding. Rivaroxaban is similar to an ideal anticoagulant. The clinical observations of secondary prophylaxis and long-term use of VTE and stroke prevention in patients with AF showed that its effectiveness and safety are at least comparable to the traditional anticoagulant, which is expected to become a novel anticoagulant drug to replace heparin and warfarin (67).
Apixaban
Apixaban was developed by Bristol-Myers Squibb with the trade name Eliquis. It was first approved by the EU in May 2011. Its indication is to prevent a VTE event in adults who were accepted elective hip or knee arthroplasty (68). In November 2012, the EU approved Apixaban to prevent stroke and systemic embolism in patients with non-valvular atrial fibrillation (NVAF) (69).
Apixaban is a highly selective and reversible inhibitor of thrombin factor Xa, which belongs to the aminobenzoxazole compounds. Therefore, it still has a good effect on treating advanced thrombosis. Compared with heparin and warfarin, the total bleeding rate of apixaban us significantly lower. This product is orally effective. In addition, apixaban is metabolized by liver and kidney, which can be used for patients with mild to moderate liver and kidney damage.
Edoxaban
Edoxaban was developed by Japan Sangyo Sankyo Co. Ltd. and was approved by the Japanese Ministry of Health and Welfare on April 22, 2011 with the trade name Lixiana. Currently, approved indications are the prevention of postoperative major orthopedic venous thromboembolism, including knee replacement surgery, hip replacement surgery and femoral hip fracture surgery. In the American Society of Hematology in Orlando (ASH) annual meeting in 2012, Sangyo Sankyo Co. Ltd. disclosed that the stage III clinical trial result of edoxaban was positive which was used in patients with hip arthroplasty replacement, and confirmed that oral edoxaban was more effective than enoxaparin in preventing VTE (70).
Comparison of characteristics of listed new anticoagulant drugs
The formation of thrombin is the central link in the blood clotting process. Direct thrombin inhibitors directly bind with the thrombin activation site to inhibit thrombin, thereby inhibiting the conversion of fibrinogen to fibrin. Direct thrombin inhibitors rarely bind with other proteins in plasma, and the anticoagulant effect is predictable.
Factor Xa occupies the starting position of the common pathway in the coagulation cascade. Selective FXa inhibitor selectively inhibits the coagulation factor which is located in the upstream of the coagulation system. It does not affect the level of existing thrombin in the body and has little effect on the basic coagulation process, reducing the risk of bleeding.
There is no direct evidence that thrombin and Xa factor which target is more advantages, but both of them have their own characteristics: Factor Xa is the first protease of the common pathway. Animal studies have shown that factor Xa inhibitors have less adverse effects on bleeding than inhibitors of factor IIa, whereas factor Xa is much less effective than factor IIa in the coagulation cascade, but factor Xa inhibition only prevents the new generation of factor IIa, which has no effect on the formed factor IIa. Thrombin direct inhibitors act more rapidly and are also effective for established factor IIa, especially in patients with high-risk thrombosis (e.g., AF) (71) (Table 6).

Full table
Conclusions
Anticoagulant market growth momentum include the increased elderly population, increased incidence of cardiovascular disease, and the new listed drugs which are significantly improved in comparison to existing drugs. According to Evaluate Pharma report, anticoagulants have the highest annual rate of growth in the top 15 treatment areas, mainly due to the increased use of Bayer’s Xarelto (rivaroxaban) and Ringer’s Pradaxa (Clopidogrel) and clesulenol (enoxaparin).As the largest market share of anticoagulant drugs—the field of stroke prevention in patients with AF, it is believed that warfarin will eventually be phased out as oral anticoagulants are introduced and infiltrated. Two of the most promising drugs in anticoagulants are inhibitors of direct coagulation factor Xa (representing drugs: rivaroxaban, apixaban, etaneraban) and direct coagulation factor IIa inhibitors (representing drug: Dabigatran etexilate). The two new oral anticoagulants are considered as a major advance in the field of anticoagulant therapy and thromboprophylaxis.
In domestic Chinese hospitals, rivaroxaban has become one of the top 5 medications sold in 2014. In the next few years, competitions in the market for rivaroxaban, dabigatran etexilate, apixaban and elixaban, which represent novel oral anticoagulant, are expected to be very intense.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Weitz JI, Eikelboom JW, Samama MM, et al. New antithromboticdrugs: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence - Based Clinical Practice Guidelines. Chest 2012;141:S120-51.
- Kroegel C, Reissig A. Principle mechanisms underlying venous thromboembolism: epidemiology, risk factors, pathophysiology and pathogenesis. Respiration 2003;70:7-30. [Crossref] [PubMed]
- Spencer FA, Emery C, Lessard D, et al. The Worcester venous thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med 2006;21:722-7. [Crossref] [PubMed]
- Fernández Capitán MC. Epidemiology of thromboembolic diseases: atrialfibrillation, venous thromboembolic disease and acute coronary syndrome. Med Clin (Barc) 2012;139:4-9. [PubMed]
- Wang C. Prevention and treatment of venous thrombosis are facing challenges. Health Care Today 2014;11:10.
- Chaudhari K, Hamad B, Syed BA. Antithrombotic drugs market. Nat Rev Drug Discov 2014;13:571-2. [Crossref] [PubMed]
- Perzborn E, Roehrig S, Straub A, et al. The discovery and development of rivaroxaban, an oral direct factor Xa inhibitor. Nat Rev Drug Discov 2011;10:61-75. [Crossref] [PubMed]
- Khoo CW, Tay KH, Shantsila E, et al. Novel oral anticoagulants. Int J Clin Pract 2009;63:630-41. [Crossref] [PubMed]
- Anderson JL, Adams CD, Antman EM, et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:e179-347. [Crossref] [PubMed]
- O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the AmericanCollege of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:78-140. [Crossref]
- Hamm CW, Bassand JP, Agewall S, et al. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2999-3054. [Crossref] [PubMed]
- Steg PG, James SK, Atar D, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). Eur Heart J 2012;33:2569-619. [Crossref] [PubMed]
- Angiolillo DJ, Capodanno D, Goto S. Platelet thrombin receptor antagonism and atherothrombosis. Eur Heart J 2010;31:17-28. [Crossref] [PubMed]
- Patrono C, García Rodríguez LA, Landolfi R, et al. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med 2005;353:2373-83. [Crossref] [PubMed]
- Capodanno D, Patel A, Dharmashankar K, et al. Pharmacodynamic effects of different aspirin dosing regimens in type 2 diabetes mellitus patients with coronary artery disease. Circ Cardiovasc Interv 2011;4:180-7. [Crossref] [PubMed]
- Muñiz-Lozano A, Rollini F, Franchi F, et al. Update on platelet glycoprotein IIb/IIIa inhibitors: recommendations for clinical practice. Ther Adv Cardiovasc Dis 2013;7:197-213. [Crossref] [PubMed]
- De Luca G, Navarese E, Marino P. Risk profile and benefits from GP IIb-IIIa inhibitors among patients with ST-segment elevation myocardial infarction treated with primary angioplasty: a meta-regression analysis of randomized trials. Eur Heart J 2009;30:2705-13. [Crossref] [PubMed]
- Storey RF, Newby LJ, Heptinstall S. Effects of P2Y1 and P2Y12 receptor antagonists on platelet aggregation induced by different agonists in human whole blood. Platelets 2001;12:443-7. [Crossref] [PubMed]
- Schömig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med 1996;334:1084-9. [Crossref] [PubMed]
- Bertrand ME, Legrand V, Boland J, et al. Randomized multicenter comparison of conventional anticoagulation versus antiplatelet therapy in unplanned and elective coronary stenting. The full anticoagulation versus aspirin and ticlopidine (FANTASTIC) study. Circulation 1998;98:1597-603. [Crossref] [PubMed]
- Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med 1998;339:1665-71. [Crossref] [PubMed]
- Urban P, Macaya C, Rupprecht HJ, et al. Randomized evaluation of anticoagulation versus antiplatelet therapy after coronary stent implantation in high-risk patients: the Multicenter Aspirin and Ticlopidine Trial After Intracoronary Stenting (MATTIS). Circulation 1998;98:2126-32. [Crossref] [PubMed]
- Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949-3003. [Crossref] [PubMed]
- Brar SS, ten Berg J, Marcucci R, et al. Impact of platelet reactivity on clinical outcomes after percutaneous coronary intervention. A collaborative meta-analysis of individual participant data. J Am Coll Cardiol 2011;58:1945-54. [Crossref] [PubMed]
- Price MJ, Berger PB, Teirstein PS, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA 2011;305:1097-105. [Crossref] [PubMed]
- Wiviott SD, Trenk D, Frelinger AL, et al. Prasugrel compared with high loading-and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation Thrombolysis In Myocardial Infarction 44 trial. Circulation 2007;116:2923-32. [Crossref] [PubMed]
- Wiviott SD, Braunwald E, McCabe CH, et al. TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001-15. [Crossref] [PubMed]
- Gurbel PA, Bliden KP, Butler K, et al. Randomized double-blind assessment of the onset and offset of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSETstudy. Circulation 2009;120:2577-85. [Crossref] [PubMed]
- FDA. Ticagrelor Full Prescribing Information, (2013-12-17) [2016-04-21]. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022433s010lbl.pdf
- EMA. Ticagrelor Assessment Report, (2013-07-21) [2016-04-21]. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001241/WC500100492.pdf
- Franchi F, Rollini F, Muñiz-Lozano A, et al. Cangrelor: a review on pharmacology and clinical trial development. Expert Rev Cardiovasc Ther 2013;11:1279-91. [Crossref] [PubMed]
- Angiolillo DJ, Schneider DJ, Bhatt DL, et al. Pharmacodynamic effects of cangrelor and clopidogrel: the platelet function substudy from the Cangrelor Versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition (CHAMPION) trials. J Thromb Thrombolysis 2012;34:44-55. [Crossref] [PubMed]
- FDA. CangrelorFull Prescribing Information, (2015-06-22) [2016-04-21]. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022433s010lbl.pdf.http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/204958lbl.pdf
- Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost 2005;3:1800-14. [Crossref] [PubMed]
- Morrow DA, Braunwald E, Bonaca MP, et al. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med 2012;366:1404-13. [Crossref] [PubMed]
- Goto S, Ogawa H, Takeuchi M, et al. Double-blind, placebo-controlled phase II studies of the protease-activated receptor 1 antagonist E5555 (atopaxar) in Japanese patients with acute coronary syndrome or high-risk coronary artery disease. Eur Heart J 2010;31:2601-13. [Crossref] [PubMed]
- O'Donoghue ML, Bhatt DL, Wiviott SD, et al. Safety and tolerability of atopaxar in the treatment of patients with acute coronary syndromes: the lessons from antagonizing the cellular effects of Thrombin–Acute Coronary Syndromes Trial. Circulation 2011;123:1843-53. [Crossref] [PubMed]
- Wiviott SD, Flather MD, O'Donoghue ML, et al. Randomized trial of atopaxar in the treatment of patients with coronary artery disease: the Lessons From Antagonizing the Cellular Effect of Thrombin–Coronary Artery Disease trial. Circulation 2011;123:1854-63. [Crossref] [PubMed]
- Capra V, Bäck M, Angiolillo DJ, et al. Impact of vascular thromboxane prostanoid receptor activation on haemostasis, thrombosis, oxidative stress, and inflammation. J Thromb Haemost 2014;12:126-37. [Crossref] [PubMed]
- Barnes NM, Sharp T. A review of central 5-HT receptors andtheirfunction. Neuropharmacology 1999;38:1083-152. [Crossref] [PubMed]
- Song Q, Zhang XW, Zhang R. Research status of the 5-hydroxytryptamine receptors and their subtype. Gansu Medical Journal 2010;29:1-4.
- Kajiwara I, Soejima H, Miyamoto S, et al. Effects of additionaltreatment of sarpogrelate to aspirin therapy on platelet aggregationand plasma plasminogen activator inhibitor activity in patientswith stable effort angina. Thromb Res 2011;128:547-51. [Crossref] [PubMed]
- Ferrandon P, Barcelo B, Perche JC, et al. Effects of dipyridamole, soluflazine and related molecules on adenosine uptake and metabolism by isolated human red blood cells. Fundam Clin Pharmacol 1994;8:446-52. [Crossref] [PubMed]
- Gotoh F, Tohgi H, Hirai S, et al. Cilostazol stroke prevention study:A placebo-controlled double-blind trial for secondary prevention ofcerebral infarction. J Stroke Cerebrovasc Dis 2000;9:147-57. [Crossref] [PubMed]
- Shinohara Y, Katayama Y, Uchiyama S, et al. Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol 2010;9:959-68. [Crossref] [PubMed]
- Labarthe B, Babin J, Bryckaert M, et al. Effects ofP2Y(1) receptor antagonism on the reactivity of plateletsfrom patients with stable coronary artery disease usingaspirin and clopidogrel. Br J Pharmacol 2012;166:221-31. [Crossref] [PubMed]
- Bird JE, Wang X, Smith PL, et al. A platelet targetfor venous thrombosis? P2Y1 deletion or antagonismprotects mice from vena cava thrombosis. J Thromb Thrombolysis 2012;34:199-207. [Crossref] [PubMed]
- Russo JJ, Goodman SG, Cantor WJ, et al. Efficacy and safety of a routine early invasive strategy after fibrinolysis stratified by glycoprotein IIb/IIIa inhibitor use during percutaneous coronary intervention: a pre-specified subgroup analysis of the TRANSFER-AMI randomised controlled trial. Heart 2014;100:873-80. [Crossref] [PubMed]
- Hsu CC, Wu WB, Huang TF. A snake venom metalloproteinase, kistomin, cleaves platelet glycoprotein VI and impairs platelet functions. J Thromb Haemost 2008;6:1578-85. [PubMed]
- Ungerer M, Rosport K, Bültmann A, et al. Novel antiplatelet drug revacept (Dimeric Glycoprotein VI-Fc) specifically and efficiently inhibited collagen-induced platelet aggregation without affecting general hemostasis in humans. Circulation 2011;123:1891-9. [Crossref] [PubMed]
- Fontayne A, Meiring M, Lamprecht S, et al. The humanized anti-glycoprotein Ib monoclonal antibody h6B4-Fab is a potent and safe antithrombotic in a high shear arterial thrombosis model in baboons. Thromb Haemost 2008;100:670-7. [Crossref] [PubMed]
- Vilahur G, Segalés E, Casaní L, et al. A novel anti-ischemic nitric oxide donor inhibits thrombosis without modifying haemodynamic parameters. Thromb Haemost 2004;91:1035-43. [PubMed]
- Heptinstall S, Espinosa DI, Manolopoulos P, et al. DG-041 inhibits the EP3 prostanoid receptor--a new target for inhibition of platelet function in atherothrombotic disease. Platelets 2008;19:605-13. [Crossref] [PubMed]
- Adams JW, Ramirez J, Shi Y, et al. APD791, 3-methoxy-n-(3-(1-methyl-1h-pyrazol-5-yl)-4-(2-morpholinoethoxy)phenyl)benzamide, a novel 5-hydroxytryptamine 2A receptor antagonist: pharmacological profile, pharmacokinetics, platelet activity and vascular biology. J Pharmacol Exp Ther 2009;331:96-103. [Crossref] [PubMed]
- Marshall AJ, Lill CL, Chao M, et al. Exploring the isoform selectivity of TGX-221 related pyrido[1,2-a]pyrimidinone-based Class IA PI 3-kinase inhibitors: synthesis, biological evaluation and molecular modeling. Bioorg Med Chem 2015;23:3796-808. [Crossref] [PubMed]
- Chen Z. Anticoagulant use of the "critical point" need to be cautious. Capital Medicine 2007;14:48.
- Zhang Q, Chen X, Zhu Z, et al. Structural analysis of low molecular weight heparin by ultraperformance size exclusion chromatography/time of flight mass spectrometry and capillary zone electrophoresis. Anal Chem 2013;85:1819-27. [Crossref] [PubMed]
- Sakuragawa N, Hasegawa H, Maki M, et al. Clinical evaluation of low-molecular- weight heparin (FR-860) on disseminated intravascular coagulation (DIC)--amulticenter co-operative double-blind trial in comparison with heparin. Thromb Res 1993;72:475-500. [Crossref] [PubMed]
- Wang WT, Hao CH, Zhao ZY, et al. New trends of research and development in the novel anticoagulant drugs. Drugs & Clinic 2011;26:10-24.
- Chen XH, Liang Y, Yang YM, et al. A new generation of anti-thrombotic drugs - Fondaparinux. Chinese Journal of Cardiology 2007;7:300-3.
- Sandor SS. Treating Thrombosis in the 21 st Century. N Engl J Med 2003;349:1762-4. [Crossref] [PubMed]
- Greinacher A, Lubenow N. Recombinant hirudin in clinical practice: focus on lepirudin. Circulation 2001;103:1479-84. [Crossref] [PubMed]
- Scatena R. Bivalirudin: a new generation antithrombotic drug. Expert Opin Investig Drugs 2000;9:1119-27. [Crossref] [PubMed]
- Berry CN, Girardot C, Lecoffre C, et al. Effects of the synthetic thrombininhibitor argatroban on fibrin- or clot-incorporated thrombin: comparison with heparin and recombinant Hirudin. Thromb Haemost 1994;72:381-6. [PubMed]
- Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmacodynamicsand pharmacokinetics of oral direct thrombin and factor Xa inhibitiorsin development. Clin Pharmacokinet 2009;48:1-22. [Crossref] [PubMed]
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versuswarfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51. [Crossref] [PubMed]
- Piccini JP, Patel MR, Mahaffey KW, et al. Rivaroxaban, an oral direct factor Xa inhibitor. Expert Opin Investig Drugs 2008;17:925-37. [Crossref] [PubMed]
- Watson J, Whiteside G, Perry C. Apixaban: first global approval. Drugs 2011;71:2079-89. [Crossref] [PubMed]
- Amin A, Stokes M, Wu N, et al. Application of randomized clinical trial data to actual practice: apixaban therapy for reduction of stroke risk in non-valvular atrial fibrillation patients. Curr Med Res Opin 2013;29:1253-61. [Crossref] [PubMed]
- Camm AJ, Bounameaux H. Edoxaban: a new oral direct factor xa inhibitor. Drugs 2011;71:1503-26. [Crossref] [PubMed]
- Becattini C, Vedovati MC, Agnelli G. Old and new oral anticoagulants for venous thromboembolism and atrial fibrillation: a review of the literature. Thromb Res 2012;129:392-400. [Crossref] [PubMed]