Peak expiratory flow among healthy children aged 5–14 years in China
Introduction
Measurement of peak expiratory flow (PEF) is of value for the management and follow-up of patients with asthma. Patients with asthma use the changes in PEF readings to help reveal how their lungs are functioning, and how well they are responding to treatment. The National Asthma Education and Prevention Program Expert Panel Reports and Global Initiative for Asthma guidelines recommend the use of PEF in the assessment and disposition of patients with asthma (1,2).
For this purpose, evaluation of observed PEF readings requires knowledge of its range in normal subjects of the same sex, age, and body size. However, normal PEF values for Chinese children have not been thoroughly investigated. To date, there have been only a few studies on normal PEF values, all of which have been geographically specific (3-10). These are therefore unlikely to be representative and generalizable to the entire population of Chinese children.
There has been increasing evidence that in addition to age, sex, and height differences for PEF, there are lung function differences among people of different races. Thus, formulas that have been derived based on European children appear not to be generalizable to Chinese children. As a large, multinational country, China is composed of 56 ethnic groups. Among them, Han Chinese account for 91.59% of the entire Chinese population (11). Thus, there is an urgent need to update equations that can accurately predict PEF for Han Chinese children.
The aim of the present study was to establish normal PEF values and develop predictive equations for Chinese children.
Methods
Subjects
This investigation was a cross-sectional study conducted from April 2010 to November 2012. Surveys were carried out among children aged 5 to 14 years at five research centers in five cities of Han Chinese: Guangzhou (in southern China), Suzhou (in eastern China), Chengdu (in southwestern China), Xi’an (in northwestern China) and Beijing (in northern China). One elementary school and one middle school from each city were randomly selected. Finally, surveys were completed for a total of 3,169 physically healthy children (1,596 boys and 1,573 girls), and participants underwent physical examination. Upon review of the questionnaires, children were excluded if they had (I) a recent disease of the respiratory tract during the last 4 weeks or history of chronic respiratory disease; (II) a history of severe respiratory disease, e.g., surgery of the thorax; (III) systemic disease influencing the respiratory tract, e.g., neuromuscular disorders or skeletal deformity; (IV) other significant diseases influencing the respiratory tract; (V) use of inhaled corticosteroids, bronchodilators or other medicines that influence the respiratory tract; and (VI) household exposure to tobacco smoke (10,12).
The methods were carried out in accordance with the approved guidelines. The study was approved by the by the local Institutional Review Board (No. 2010LW025). The parents of all study participants gave both verbal and written informed consent before study enrollment.
Measurement
Demographic information including age, sex, and smoking status (for children entering puberty) was collected (13). Anthropometric values (height and weight) were measured, preferably in the morning, by a physician using a standardized procedure (14). Standing height (in centimeters) was measured to the nearest 0.1 cm without shoes. Weight (in kilograms) was rounded to the nearest 100 g with the children wearing only their usual school uniforms. Height and weight were measured twice and the average used for analysis.
Before our investigation, one experienced technician from each of the five research centers received strict training, to ensure the unity of standards. The children performed flow-volume curve maneuvers in a pulmonary function laboratory at each research center, using a Jaeger Master Lab spirometer (Erich Jaeger GmBh, Wurzburg, Germany) and following guidelines of the American Thoracic Society (ATS) to ensure quality (14). Prior standard instruction was provided and each effort was coached by an experienced technician in a dedicated pediatric pulmonary clinic. Spirometry was performed in the seated position with a nose clip. Each effort in each spirometry test session was evaluated by a single investigator to determine acceptability and repeatability based on criteria of the 2007 ATS and European Respiratory Society (ERS) statement in pulmonary function testing among preschool children. Children were excluded if the flow-volume curve had an abnormal shape (14,15).
The children were then instructed in use of the Airmed mini peak flow meter (Clement Clarke International Limited, Harlow, UK), which has been designed to measure PEF. Calibration of the instrument was checked initially and periodically thereafter, using a standard peak flow meter as reference. Five mini peak flow meters were used at each research center. Agreement between the meters was within 20 L/min; this was checked on 20 people. At least one parent also attended the instruction sessions. When participants had understood the technique, and were able to perform the test correctly, they were asked to make six maximal efforts in the standing position. They were closely observed to ensure that they maintained an airtight seal between the lips and mouthpiece of the instrument. At each center, all instructions were given by the same experienced technician, who encouraged the children so as to obtain optimal lung function values.
Analysis
The data were analyzed using International Business Machines (IBM) SPSS (IBM Corp., Armonk, NY, USA). Continuous variables, such as age, were reported as mean ± standard deviation, and categorical variables, such as sex, as the percentage in each subgroup. Means for age, height, weight, forced expiratory volume in 1 second (FEV1) and PEF were stratified by sex. The t-test was used to test for differences in measurements between males and females. Correlation coefficients were calculated to examine relationships among the independent variables and PEF. Multiple linear regression analysis was used to predict PEF values. Stepwise procedures were used with the independent variables of height and weight as well as their square, cube, and natural logarithm values, to find subset factors considered to be potentially useful predictors. PEF values were calculated using the previous regression equation for Chinese children [5.29× height (cm) −427.1 for boys and 4.94× height (cm) −399.8 for girls] (16); these were then compared with the current PEF values. Absolute error was calculated as magnitude of the difference between the exact value and predictive value. Comparisons with other reference data for Irish (17), Turkish (18), British (19), Greek children (20), and Danish children (21) children were also made to show differences among the reference values for children. A P value of <0.05 was regarded as significant.
Results
A total of 3,169 healthy children from five centers in five cities throughout China were selected for the present study (Table 1). There were no significant differences in participants’ height and weight between the five centers (both P>0.05). Distributions of age and anthropometric data by sex of the study population are shown in Table 2. There were no significant differences between boys and girls in body height and weight from ages 5 to 12 years. Heights and weights were both significantly higher for boys than for girls from ages 13 to 14 years (P<0.05).
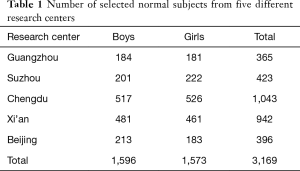
Full table
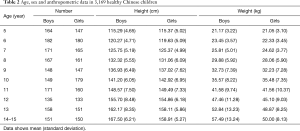
Full table
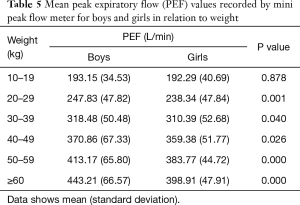
Mean PEF values recorded by mini peak flow meter in boys were significantly higher than those in girls (313.65±91.27 vs. 295.65±79.61 L/min). Mean PEF values by mini peak flow meter according to age, height, and weight for both sexes are shown in Tables 3-5. There were significant differences in PEF values between boys and girls in each age group, except for ages 5, 10, and 11 years (Table 3). Additionally, there were significant differences in PEFs between boys and girls in each height group, except for 100, 110, 140, and ≥170 cm (Table 4). There were also significant differences in PEF values between boys and girls in each weight group, except the 10-kg group (Table 5).
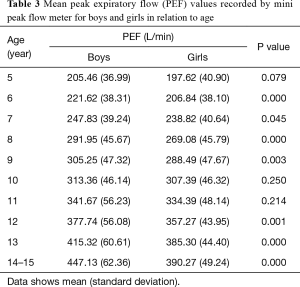
Full table
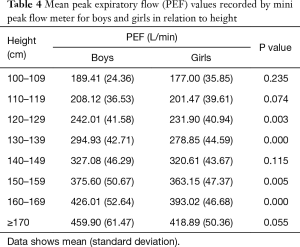
Full table
Full table
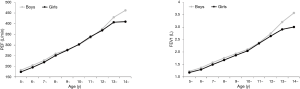
For both sexes, PEFs recorded by mini peak flow meter increased with age, height, and weight (Figure 1). The correlation coefficients between PEF values obtained by mini peak flow meter and height, weight, and age are given in Table 6. All correlations were found to be positive and significant (P<0.001).


Full table
In developing the multiple regression model, we included age, height and weight, as well as their square, cube, and natural logarithm values; height was the only significant predictor of PEF values by mini peak flow meter in the sex-specific model. Therefore, only height was used in the reference equations for PEF recorded by mini peak flow meter. The regression equation for boys was calculated as follows: PEF recorded by mini peak flow meter =4.39× height (cm) −300.48 (R2 =0.76, P<0.001); for girls this was PEF =4.13× height (cm) −278.04 (R2 =0.72, P<0.001). The scatter plots and regression lines of PEF by mini peak flow meter based on height for boys and girls are shown in Figure 2.

The mean FEV1 and PEF values recorded by spirometer according to age for boys and girls are shown in Figure 3. PEF by mini peak flow meter and PEF and FEV1 by spirometer showed a very high correlation (Figure 4); the correlation coefficient for PEF values between the two was 0.93. The correlation coefficient for PEF recorded by mini peak flow meter and FEV1 recorded by spirometer was 0.88.

PEF values were calculated using the previous regression equation used for Chinese children [5.29× height (cm) −427.1 for boys and 4.94× height (cm) −399.8 for girls]; these were then compared with the PEF values in our study (16). The mean absolute error for boys was 37.45 L/min, and mean absolute percentage error was 12.6%.
We also compared mean PEF values of Chinese children for each age and sex with those of Irish (N=2,828), Turkish (N=2,791), British (N=339), and Greek children (N=522), and for height with those of Irish, Turkish, and Danish (N=861) children. We found that PEF values of Chinese children for both sexes according to age were close to those of Irish, Turkish, and British children whereas they were lower than those of Greek children (Figure 5). PEF values according to height were similar to those of Turkish and Danish children, whereas they were lower than those of Irish children (Figure 6).


Discussion
PEF is primarily a measure of the patency of large intrathoracic airways and is used worldwide for the treatment and follow-up of asthma in children and adults (1,22,23). PEF recorded by mini peak flow meter is considered important for the screening and diagnosis of asthma in population-based studies, as well as for assessing disease severity and prognosis. This measure is simple to perform, easily learned, and reproducible. Therefore, it is necessary to develop predictive equations for PEF.
Reliable normal reference value does rely on appropriate sample size, nevertheless, due to technological difficulty, it is impossible to generate the reference value from a randomized community sample, and it is also difficult to predict the proper sample size. We searched more than 30 published references and only found one paper recommend the sample size estimation. Quanjer et al. had discussed the influence of sample size on lung function reference equations, and suggested that at least 300 local healthy subjects (150 males and 150 females) would be needed to validate reference values to avoid spurious differences due to sampling error (24). In our study, the sample size is more than 3,000 (each research center has a sample size more than 300), therefore we think our sample size is big enough to generate a reliable normal reference for PEF.
Variables of ethnicity, sex, age, weight, and height are known to have an impact on PEF values (25). In addition, environmental factors affecting growth and development, such as child health nutritional status and air quality, may change over long periods (26); therefore, PEF values for a given ethnic community may change over time. To the best of our knowledge, there are only seven studies in the literature that used predictive equations for PEF in Chinese children (3-10); however, these studies were all geographically specific. Although no differences were found between different areas of China, the results of these studies are unlikely to be representative and generalizable to the entire population of children in China (6). Hence, it is crucial to develop equations to accurately predict PEF for Chinese children.
Our study used stepwise multiple regression techniques to predict PEF using predictor variables of height, weight and age, as well as their square, cube, and natural logarithm values, stratified by sex. We used simple reference equations for both boys and girls based on height alone for Chinese children. We found differences in the PEF values between boys and girls. We determined scatter plots and regression lines of PEF recorded by mini peak flow meter based on height in boys and girls. We believe that charts of PEF plotted against height can be easily used by respiratory physicians to assess the lung function of a given child.
We made comparisons between PEF recorded by mini peak flow meter, and PEF and FEV1 recorded by spirometer. We found that the mini peak flow meter can provide reproducible FEV1 values that are of acceptable quality. FEV1 is considered the gold standard of peripheral airway obstruction. This highlights the importance of the mini peak flow meter when hospital spirometry is unavailable.
We made comparisons with other reference data for Irish (17), Turkish (18), British (19), Greek children (20), and Danish children (21) children to show differences among the reference values for children. We found that PEF values for Chinese children of both sexes according to age were close to those of Irish, Turkish, and British children but were lower than those of children in Greece; PEF values according to height were similar to those of Turkish and Danish children but lower than values for children in Ireland. These differences may be owing to the methodology used, sampling variability, and ethnic differences in body morphology and lung dimension and function. Relatively small sample sizes may also cause increased random variability.
The current study has some limitations. First, the developed predictive equations are only applicable to the age-specific population of Chinese children from 5 to 14 years old. Because a child in China is defined as being below age 14 years, participants older than 14 years of age were not included. Further studies are needed among Chinese children younger than 5 years old, to better understand pulmonary function in children of different ages. Second, using different spirometric technicians in the different settings could be a source of increased data variance. However, all technicians performed spirometric measures according to guidelines and requirements of the ATS/ERS; thus, different technicians should not affect greatly affect the measurements. Lastly, our use of age in whole years could be limited compared with more precise decimal age.
In conclusion, our study established normal values of PEF and developed predictive equations for PEF using linear regression analysis for children aged 5–14 years in China.
Acknowledgements
Funding: Research reported in this publication was supported by Chinese Medical Association research projects, National Key Technology R&D Program (to Y Gao, No. 2015BAI12B10); Provincial Key Research and Development Program of Jiangsu (No. SBE2016720004); Key Projects for Social Development of Jiangsu Province (No. BE2017657); Science and Technology Projects of Suzhou (No. SS201646).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the by the local Institutional Review Board (No. 2010LW025). The parents of all study participants gave both verbal and written informed consent before study enrollment.
References
- Global Initiative for Asthma. Global Strategy for asthma management and prevention. Accessed May 6, 2015. Available online: http://www.ginasthma.com
- National Asthma Education and Prevention Program. National Asthma Education and Prevention Program. Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma Update on Selected Topics--2002. J Allergy Clin Immunol 2002;110:S141-219. [PubMed]
- Ip MS, Karlberg EM, Chan KN, et al. Lung function reference values in Chinese children and adolescents in Hong Kong. II. Prediction equations for plethysmographic lung volumes. Am J Respir Crit Care Med 2000;162:430-5. [Crossref] [PubMed]
- Ip MS, Karlberg EM, Karlberg JP, et al. Lung function reference values in Chinese children and adolescents in Hong Kong. I. Spirometric values and comparison with other populations. Am J Respir Crit Care Med 2000;162:424-9. [Crossref] [PubMed]
- Jeng MJ, Chang HL, Tsai MC, et al. Spirometric pulmonary function parameters of healthy Chinese children aged 3-6 years in Taiwan. Pediatr Pulmonol 2009;44:676-82. [Crossref] [PubMed]
- Jiang M, Gao Y, Zhong NS, et al. Spirometric reference values for healthy Han children aged 5-15 years in Guangzhou, southern China. Pediatr Pulmonol 2015;50:1009-16. [Crossref] [PubMed]
- Tsai MC, Jeng MJ, Chang HL, et al. Spirometric reference equations for healthy children aged 6 to 11 years in Taiwan. J Chin Med Assoc 2010;73:21-8. [Crossref] [PubMed]
- Feng K, Chen L, Han SM, et al. Spirometric standards for healthy children and adolescents of Korean Chinese in northeast China. J Korean Med Sci 2011;26:1469-73. [Crossref] [PubMed]
- Ma YN, Wang J, Dong GH, et al. Predictive equations using regression analysis of pulmonary function for healthy children in Northeast China. PLoS One 2013;8:e63875. [Crossref] [PubMed]
- Leung TF, Liu TC, Mak KK, et al. Reference standards for forced expiratory indices in Chinese preschool children. Pediatr Pulmonol 2013;48:1119-26. [Crossref] [PubMed]
- Chu JY, Huang W, Kuang SQ, et al. Genetic relationship of populations in China. Proc Natl Acad Sci U S A 1998;95:11763-8. [Crossref] [PubMed]
- Brouwer AF, Roorda RJ, Duiverman EJ, et al. Reference values for peak flow and FEV1 variation in healthy school children using home spirometry. Eur Respir J 2008;32:1262-8. [Crossref] [PubMed]
- Brazzale D, Hall G, Swanney MP. Reference values for spirometry and their use in test interpretation: A Position Statement from the Australian and New Zealand Society of Respiratory Science. Respirology 2016;21:1201-9. [Crossref] [PubMed]
- Standardization of Spirometry. 1994 Update. American Thoracic Society. Am J Respir Crit Care Med 1995;152:1107-36. [Crossref] [PubMed]
- Lum S, Bountziouka V, Sonnappa S, et al. How "healthy" should children be when selecting reference samples for spirometry? Eur Respir J 2015;45:1576-81. [Crossref] [PubMed]
- Zhong NS. Normal values of peak expiratory flow and its application in asthma. Chin J Tubere Respir Dis 1985;8:138.
- Carson JW, Hoey H, Taylor MR. Growth and other factors affecting peak expiratory flow rate. Arch Dis Child 1989;64:96-102. [Crossref] [PubMed]
- Ones U, Somer A, Sapan N, et al. Peak expiratory flow rates in healthy Turkish children living in Istanbul, Turkey. Allergy Asthma Proc 2004;25:313-20. [PubMed]
- Primhak RA, Biggins JD, Tsanakas JN, et al. Factors affecting the peak expiratory flow rate in children. Br J Dis Chest 1984;78:26-35. [Crossref] [PubMed]
- Tsanakas JN, Primhak RA, Milner RD, et al. Unexpectedly high peak expiratory flow rates in normal Greek children. Eur J Pediatr 1983;141:46-9. [Crossref] [PubMed]
- Høst A, Høst AH, Ibsen T. Peak expiratory flow rate in healthy children aged 6-17 years. Acta Paediatr 1994;83:1255-7. [Crossref] [PubMed]
- International consensus report on diagnosis and treatment of asthma. National Heart, Lung, and Blood Institute, National Institutes of Health. Bethesda, Maryland 20892. Eur Respir J 1992;5:601-41. [PubMed]
- Quanjer PH, Lebowitz MD, Gregg I, et al. Peak expiratory flow: conclusions and recommendations of a Working Party of the European Respiratory Society. Eur Respir J Suppl 1997;24:2S-8S. [PubMed]
- Quanjer PH, Stocks J, Cole TJ, et al. Influence of secular trends and sample size on reference equations for lung function tests. Eur Respir J 2011;37:658-64. [Crossref] [PubMed]
- Takase M, Sakata H, Shikada M, et al. Development of reference equations for spirometry in Japanese children aged 6-18 years. Pediatr Pulmonol 2013;48:35-44. [Crossref] [PubMed]
- Leung SS, Lau JT, Xu YY, et al. Secular changes in standing height, sitting height and sexual maturation of Chinese--the Hong Kong Growth Study, 1993. Ann Hum Biol 1996;23:297-306. [Crossref] [PubMed]


