Early radiological response as predictor of overall survival in non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor mutations
Introduction
Lung cancer is the leading cause of cancer-related death in worldwide, with higher mortality rates than any other type of cancer (1). Approximately 85% is non-small cell lung cancer (NSCLC). Most patients present with advanced or metastatic disease, where the surgery is not indicated. Furthermore, a significant proportion of patients diagnosed with early-stage NSCLC eventually experience metastatic disease or regional relapse (1,2).
Platinum-based doublet chemotherapy combinations, which have represented the cornerstone of the initial treatment for most patients with advanced NSCLC, confer a survival benefit which is equivalent to a 9% absolute improvement in 1-year survival rates (increasing survival from 20% to 29%) (3). In the last decade, the identification of molecular subtypes of NSCLC has significantly modified the management and prognosis of a subset of patients. The discovery of epidermal growth factor receptor (EGFR) mutations and ALK/ROS1 translocations (4-9), and the significant progression-free survival (PFS) advantage of EGFR (erlotinib, gefitinib, afatinib) or ALK/ROS1 (crizotinib) inhibitors over chemotherapy in this subpopulation in large randomized trials (6-13) has led to a biomarker-based approach for the selection of first-line therapy. Additionally, anti-PD-1/PD-L1 agents have shown higher efficacy compared with chemotherapy, firstly in second-line setting and lately as first-line therapy in patients with metastatic NSCLC with a ≥50% PD-L1 expression (14-18). Therefore, currently patients with EGFR mutations or ALK/ROS1 translocations are eligible for treatment with EGFR or ALK/ROS1 tyrosine kinase inhibitors (TKIs), respectively, while patients without a known driver mutation generally receive treatment with immunotherapy (if ≥50% PD-L1 expression) or platinum-doublet chemotherapy combinations (if <50% PD-L1 expression) (14,15).
Despite the significant response rates (RRs) observed with EGFR TKIs in EGFR positive tumors, response to therapy is not uniform (19) and identifying patients that will respond to therapy remains a challenge. In patients receiving cytotoxic agents, objective tumor regression measured as overall response rate (ORR) has been used as primary efficacy endpoint in drug development, and radiologic assessment following Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 criteria, is currently recommended after the completion of two or three cycles of chemotherapy (19,20). However, the mechanism of action of molecular targeted agents such as EGFR TKIs is different, raising questions as to whether RECIST version 1.1 can serve as an appropriate efficacy endpoint. Moreover, the optimal timing for response evaluation of EGFR TKI is not well-defined, since response can be observed as early as a few days after treatment initiation (16,19,21-24).
We performed a retrospective, single centre study to evaluate the association between early radiological response (ERR) with survival in EGFR-mutated NSCLC patients treated with first- or second-generation EGFR TKIs.
Methods
Study design
Locally advanced (no suitable for radical treatment) or metastatic NSCLC EGFR mutated patients diagnosed between January 2009 and November 2014 in the Hospital Universitari i Politècnic La Fe in Valencia (Spain) were included in this study. Eligibility criteria included: (I) histologically confirmed NSCLC with locally advanced or metastatic disease (stage IIIB or IV); (II) presence of activating EGFR mutation; (III) ≥18 years old; (IV) Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2; and (V) treatment with EGFR TKIs gefitinib, erlotinib or afatinib once daily at least for 2 weeks.
Response to EGFR TKI therapy was defined according to RECIST version 1.1 (24). ERR was defined as complete response (CR) or partial response (PR) at first radiographic evaluation performed after 6–8 weeks from the beginning of treatment.
Initial patient evaluation included a complete blood count, biochemical evaluation [albumin, renal function, liver enzymes, lactate dehydrogenase (LDH)], an electrocardiogram, a chest X-ray and computed tomography (CT)-scan for evaluation of disease extension.
Clinical data were derived from review of electronic medical records. Variables considered in our study were: sex, age, race, smoking, disease stage, histological type, presence of metastasis and their location, type of treatment, chemotherapy and its characteristics (first and successive treatment lines), treatment with TKI, toxicities, treatment delays due to toxicity, supportive treatment. Adverse events were graded according to National Cancer Institute Common Toxicity Criteria version 4.
Tissue and EGFR analysis
Initial histological diagnosis was performed on formalin-fixed paraffin-embedded (FFPE) tissue. Five µm thick sections were macrodissected by a pathologist to select regions containing the highest proportion of tumor cells. Genomic DNA was isolated from FFPE sections using Deparaffinization Solution and QIAamp DNA Investigator Kit (Qiagen, Hilden, Germany) according to manufacturer’s protocol. DNA concentration was quantified by spectrophotometer using NanoDrop 2000c (ThermoFisher Scientific, Waltham, MA, USA). Samples were tested by real time polymerase chain reaction (PCR) in a cobas z480 analyzer using the cobas®EGFR Mutation Test v2 (CE-IVD; Roche Molecular Diagnostics, Basel, Switzerland), which can detect 42 mutations in exons 18–21 of the EGFR gene.
Statistical analysis
A descriptive analysis of patient characteristics, treatments administered and their most relevant toxicities was performed. Treatment response (TR) rates, toxicities, PFS and overall survival (OS) rates were estimated as proportions (%); comparisons between proportions were estimated using the Chi-square test. PFS and OS were estimated by the Kaplan-Meier method and differences among the different groups were analyzed with log-rank test (P values below 0.05 were considered statistically significant).
Univariate Cox hazards regression models were used to evaluate the association of smoking, PS, ERR, line of treatment and type of EGFR mutation with OS. Variables with a P value <0.1 in univariate analysis were selected for inclusion in the multivariate model. Additionally, investigators decided to include mutation type in the multivariate model despite it not reaching a significant P value due to its clinical relevance. Multivariate Cox hazards regression analysis was used to evaluate independent prognostic factors associated to OS. ERR, line of treatment and type of EGFR mutation were used as covariates. Variables with a P value lower than 0.05 were considered to indicate statistical significance. Statistical analysis and graphical representations were performed using SPSS statistics v19 (IBM, Armonk, NY, USA).
Results
Patients’ characteristics
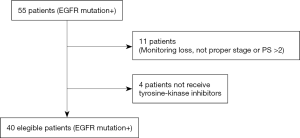
Between January 2009 and November 2014, EGFR mutation status was analysed in 360 NSCLC patients. Fifty-five patients (55/360; 15.3%) were EGFR mutation positive and 40 of them met inclusion criteria (Figure S1). The clinical and pathologic characteristics of the 40 eligible patients are summarized in Table 1. EGFR mutations identified in the tumors, were mainly exon 19 deletions (17/40 patients; 42.5%), and L858R point mutation (16/40 patients; 40.0%). Median follow-up was 21 months (range, 2–75 months). At the moment of the analysis, 25 patients (62.5%) had died.
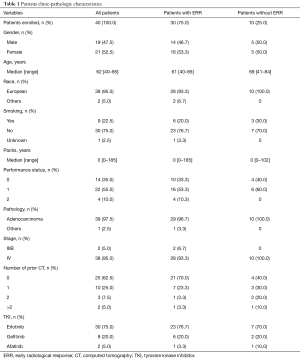
Full table
TKI response and survival
Thirty patients (75%) experienced an ERR; of these, 4 experienced a CR and 26 had a PR. Of those patients with a PR as their ERR, 4 eventually experienced a CR on subsequent radiographic assessments. Ten patients (25%) did not experience an ERR; of these, 5 experienced stable disease (SD) and 5 experienced progressive disease (PD). None of the patients that did not achieve an ERR experienced any type of response (PR or CR) on subsequent radiographic assessments. Oligoprogression was not observed in any patient.
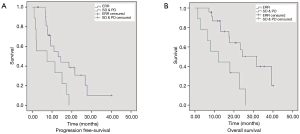
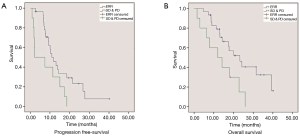
Median PFS was significantly longer in patients experiencing an ERR (CR or PR) (10.9 vs. 2.4 months; HR: 0.42; 95% CI: 0.19–0.93; P=0.033) than those with SD or PD (Figure 1). Median OS was also significantly increased in patients experiencing an ERR (23.9 vs. 11.9 months; HR: 0.3; 95% CI: 0.15–0.85; P=0.021).
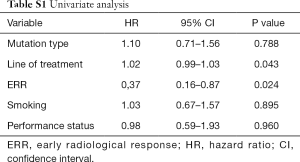
On univariate analysis, ERR and line of treatment were the only variables significantly associated with OS (Table S1). Although the type of mutation did not reach a significant P value on univariate analysis, the authors decided to include it in the multivariate analysis due to its clinical relevance. In the multivariate Cox hazards regression model, only ERR remained as an independent variable (HR: 0.36; 95% CI: 0.16–0.86; P=0.021) (Table S2).
Full table
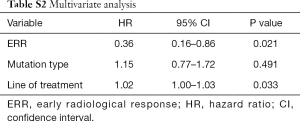
Full table
EGFR mutation subtypes and survival
We also evaluated the relationship between treatment efficacy and the different EGFR-mutation subtypes. The ERR rate was 76.5% (13/17) in patients harboring exon 19 deletions and 69% (11/16) in patients bearing L858R point mutations; no significant differences were observed between groups (P=0.619). When considering only those patients with activating EGFR mutations (exon 19 deletions or L858R point mutations), a similar survival benefit in those experiencing an ERR was observed. Median PFS (13 vs. 7 months; HR: 0.368; 95% CI: 0.154–0.881; P=0.025) and OS (24 vs. 12 months; HR: 0.278; 95% CI: 0.106–0.731; P=0.009) was significantly longer in patients that experienced an ERR than those experiencing SD or PD (Figure 2).
Treatment-related adverse effects
The most frequent treatment-related adverse effects were grade 1–2 skin toxicity (rash and dryness) in 59% of patients. Treatment was interrupted in 5 patients (13%) because of skin toxicity (rash). Dose reduction was required in 8 patients (20%) due to skin toxicity (n=4; 10%) and worsening of general condition (n=4; 10%).
Discussion
EGFR activating mutations represent the first molecular biomarker implemented for clinical use in lung cancer, leading to the use of TKIs as standard first line therapy in a subset of patients. Although radiographic response criteria (RECIST v1.1) evaluated after 2–3 cycles of chemotherapy is a well-established efficacy end-point for cytotoxic chemotherapy, its validity as an efficacy biomarker in patients treated with TKIs is less well recognized.
The optimal time-point for the initial response evaluation to TKIs remains a matter of debate. Most pivotal trials that comparing TKIs to chemotherapy used the first response evaluation performed after 6–8 weeks of treatment (6,7,21,22) as the efficacy endpoint. However, response to therapy as early as days after treatment initiation has been reported (19). Some studies have tried to correlate responses at earlier time-points with the outcome of TKI therapy in patients with NSCLC and EGFR mutations (4-26). Chang et al. reported an association between the early radiological image change and the overall radiographic response, although the difference was not statistically significant, possibly due to the reduced number of patients enrolled (19). An association between ERR, measured by positron emission tomography (PET), with RECIST response in patients treated with gefitinib and erlotinib has been reported (23-25); the role of PET in this context must, however, still be determined (23-25). In other clinical scenarios, such as neoadjuvant chemotherapy in patients with locally advanced tumors, an association between radiographic response and survival has also been reported (24,26).
The clinical characteristics of patients participating in our study are similar to those reported in previous studies evaluating EGFR mutant NSCLC cohorts, except for a similar proportion of women and men observed in our cohort. Treatment efficacy, despite the fact most patients have received one or more previous treatments, was similar to previously published studies (6-8).
In our group of EGFR-mutant NSCLC patients treated with TKIs, ERR was found to be associated with significantly higher median PFS and OS (10.9 vs. 2.4 months and 23.9 vs. 11.9 months, respectively) than patients who achieved SD or PD at first radiographic evaluation. Our results confirm the association between RR and OS, that has been reported in a systematic review of 28 trials with EGFR TKI monotherapy (27), as well as observations previously reported by Chang et al. (19), whereby patients with activating EGFR mutations experience proportionally higher ORR to EGFR-TKI treatment. In our study, the difference in survival observed in the subgroup of patients harboring EGFR activating mutations that experienced ERR and those with SD or PR was even larger than in the general population.
Overall, the results of this study suggest that patients treated with TKI that do not respond at the first evaluation have a worse prognosis, which is independent of the presence of EGFR mutations, with PFS and OS values that are similar to those reported for patients treated with chemotherapy (16). Close monitoring of these patients in order to detect an early progression could potentially enable a switch to alternative treatment options before clinical progression occurs.
Limitations of our study include the heterogeneity of the population, with different types and lines of treatments, the small sample size and the retrospective character of the analysis. The presence of T790M mutations at progression was not clinically assessed in our centre during the times when most of participants were treated. Moreover, no patients in the study experimented oligoprogression. Therefore, our results cannot be extrapolated to patients experiencing oligoprogression at an early radiographic assessment while on EGFR TKIs. All these issues should be considered in future prospective studies.
In conclusion, ERR may serve as an early indicator of TKI inhibitor efficacy in EGFR mutant NSCLC patients. Although the early identification of non-responders could allow an early treatment switch while PS is still optimal, which could increase the efficacy of subsequent lines of treatment, continuation of treatment with TKIs is currently recommended in cases of oligoprogressive disease (28). Data from prospective cohorts will be needed to elucidate the value of early responses in patients treated with EGFR TKIs, in order to maximize outcome.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics committee of “Comité Ético de Investigación Biomédica Hospital La Fe” (No. 2015/0345).
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Thomas A, Rajan A, Giaccone G. Tyrosine Kinase Inhibitors in Lung Cancer. Hematol Oncol Clin North Am 2012;26:589-605. [Crossref] [PubMed]
- NSCLC Meta-Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol 2008;26:4617-25. [Crossref] [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non small cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Paez JG, Jänne PA, Lee JC, et al. EGFR Mutations in Lung Cancer: Correlation with Clinical Response to Gefitinib Therapy. Science 2004;304:1497-500. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866-74. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase III trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Mazières J, Zalcman G, Crinò L, et al. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol 2015;33:992-9. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Barlesi F, Steins M, Horn L, et al. Long-term outcomes with nivolumab (Nivo) vs docetaxel (Doc) in patients (Pts) with advanced (Adv) NSCLC: CheckMate 017 and CheckMate 057 2-y update. Ann Oncol 2016;27:abstr 1215PD.
- Chang JW, Hou MM, Hsieh JJ, et al. Early Radiographic Response to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor in Non small Cell Lung Cancer Patients with Epidermal Growth Factor Receptor Mutations: A Prospective Study. Biomed J 2015;38:221-8. [Crossref] [PubMed]
- Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v1-27. [Crossref] [PubMed]
- Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 2012;30:1122-8. [Crossref] [PubMed]
- Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet 2008;372:1809-18. [Crossref] [PubMed]
- Sunaga N, Oriuchi N, Kaira K, et al. Usefulness of FDG PET for early prediction of the response to gefitinib in non small cell lung cancer. Lung Cancer 2008;59:203-10. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Ullrich RT, Zander T, Neumaier B, et al. Early detection of erlotinib treatment response in NSCLC by 3’ deoxy 3’ [F] fluoro L thymidine ([F] FLT) positron emission tomography (PET). PLoS One 2008;3:e3908. [Crossref] [PubMed]
- Tanvetyanon T, Eikman EA, Sommers E, et al. Computed tomography response, but not positron emission tomography scan response, predicts survival after neoadjuvant chemotherapy for resectable non-small-cell lung cancer. J Clin Oncol 2008;26:4610-6. [Crossref] [PubMed]
- Tsujino K, Kawaguchi T, Kubo A, et al. Response Rate Is Associated with Prolonged Survival in Patients with Advanced Non-small Cell Lung Cancer Treated with Gefitinib or Erlotinib. J Thorac Oncol 2009;4:994-1001. [Crossref] [PubMed]
- Yap TA, Macklin-Doherty A, Popat S. Continuing EGFR inhibition beyond progression in advanced non-small cell lung cancer. Eur J Cancer 2017;70:12-21. [Crossref] [PubMed]


