Less invasive HeartMate 3 left ventricular assist device implantation
Introduction
With the growing numbers of patients diagnosed with advanced stage heart failure and a growing acceptance of device therapy, the application of left ventricular assist devices (LVADs) is increasing worldwide (1-12). Technological developments and increased surgical experience has led to significant improvement of circulatory support with LVADs. One important design characteristic is miniaturization of the implanted blood pump, which provides the opportunity to reduce surgical trauma during implantation with limited approach-deployment (2).
The HeartMate 3 LVAD (Abbott, Chicago, IL, USA), was first implanted in June 2014 and received CE Mark approval on the basis of excellent results in a clinical trial completed in 2015 (1). The HeartMate 3 is a compact LVAD that offers several new technical features, including a fully magnetically levitated rotor, large blood-flow gaps, textured blood-contacting surfaces, and an artificial pulse. These features are designed to improve hemocompatibility by minimizing shear-induced damage to blood components. The small size of the HeartMate 3 and its integral inflow cannula requires intrathoracic placement, eliminating the need for an abdominal pocket. Furthermore, this configuration enables minimal-invasive implantation, which is possibly associated with decreased perioperative complications, such as right heart failure, bleeding, and wound infections (2). Herein we describe our first experiences with the technique of less-invasive HeartMate 3 implantation by upper hemi-sternotomy combined with anterior-lateral thoracotomy.
Methods
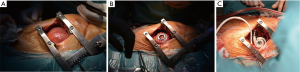
Standard cardiovascular anesthesia procedures including cardiopulmonary bypass support are employed during this operation. Transesophageal echocardiography (TEE) is used pre- and intra-operatively to identify left atrial or ventricular thrombi or any intracardiac defects, such as patent foramen ovale. The patient is placed in the supine position with the left side elevated. Cannulation for cardiopulmonary bypass is accomplished by placement of an inflow cannula into the right femoral vein, and the arterial return cannula is inserted into the distal ascending aorta through an upper hemi-sternotomy. An anterior-lateral thoracotomy through the fifth or sixth intercostal space is performed to expose the apex of the heart (Figure 1A), while an upper hemi-sternotomy down to the second or third intercostal space is concurrently performed by a second surgeon. A largely intact pericardium is maintained to avoid right ventricular dilation and failure after LVAD support is begun. Optimal left ventricular (LV) apical attachment for an inflow cannula insertion is identified by combining TEE guidance and external compression (needle, surgical instrument or finger to allow for positioning within long axis parallel to the intraventricular septum) aiming towards a mitral orifice. The HeartMate 3 apical cuff is attached to the epicardial surface at the LV apex either by interrupted pledgeted sutures or a continuous running suture (Figure 1B,C). The suture line around the apical cuff can be sealed with surgical tissue glue to minimize bleeding. Heparin is given according to standard procedures, cardiopulmonary bypass is started and entry into the left ventricle is made by coring the myocardium through the center of the apical cuff with circular knife. Under specific conditions, such as a calcified apex, excessive trabeculation or suspected intracavitary thrombus, a technique of coring the apex first, followed by LV cavity inspection and subsequent apical cuff attachment is desirable, and is the preferred technique by the authors. In this situation the authors recommend epicardial back stitching to optimize the inflow cannula apposition and hemostatic efficiency as demonstrated by in vitro-testing (4).
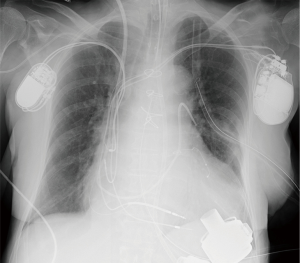
The HeartMate 3 pump is passed through the thoracotomy incision, with the inflow conduit inserted into the left ventricle through the apical cuff, and the pump is secured by a novel slide locking mechanism. The outflow graft is tunneled intrapericardially to the upper hemi-sternotomy and then anastomosed end-to-side to the ascending aorta. The driveline is passed in the sheath of the rectus muscle toward the umbilicus and then subcutaneously to the left or right upper quadrant of the abdomen and externalized with a silicon-skin interface. The pump is de-aired through the outflow graft, LVAD support is started at a low rate of speed, and cardiopulmonary bypass support is terminated. After cardiopulmonary bypass cannulas are removed, the upper hemi-sternotomy and thoracotomy incisions are closed in standard fashion. The final position of the pump is checked by intraoperative performance of TEE and postoperative chest X-ray (Figure 2).
Results
Less-invasive LVAD implantation through upper hemi-sternotomy combined with anterior lateral thoracotomy has several potential positive effects (2). The minimized surgical approach may allow diminished surgical trauma, less postoperative bleeding, maintenance of the chest stability, reduced need of blood product transfusion, and earlier recovery. The pericardium also remains intact, which helps stabilize right ventricular function by avoiding right ventricular dilation during onset of the LVAD support and thereby possibly reducing the incidence of postoperative right heart failure (5). A proclivity to sternal wound healing complications, particularly in diabetic or elderly destination therapy populations, may be ameliorated by this strategy. In the case of LVAD exchange, less-invasive approaches without sternotomy are applicable and reduce the surgical time as well as surgical wound size (6).
There are some limitations of this less invasive approach. Visual control of the heart for distention or decompression after commencing LVAD support is limited. This underscores the importance of thorough echocardiographic assessment and hemodynamic monitoring of pump speed. The estimation and trimming of proper outflow graft length also becomes more challenging. Excess outflow graft length could result in kinks, alter flow and contribute to thrombus formation. Conversely, insufficient length may compress right ventricle and compromise LVAD preload. Exposure of the apex after the pump is secured is challenging, making the control of bleeding around the apical cuff very important. Hence, application of glue helps to minimize bleeding at the apex. Tricuspid valve repair/replacement or closure of a patent foramen ovale cannot be performed with this technique.
Centrifugal pump implantation by this novel approach provides a potential for optimizing hemodynamic stability, even though it might be technically more challenging than sternotomy. Therefore, surgeons performing minimally invasive LVAD implantation should be well experienced with the use of similar implantable assist devices to ensure a good outcome. In case the outflow graft anastomosis to the ascending aorta is not feasible, alternative approaches, such as anastomoses of the outflow graft to the descending aorta are technically feasible (7).
Conclusions
Less-invasive implantation of the HeartMate 3 is therefore technically feasible, offers several benefits for surgical outcome and may become the standard of care for LVAD implantation techniques (8-12). Future comparative studies between the techniques are warranted.
Acknowledgements
None.
Footnote
Conflicts of Interest: JD Schmitto, T Krabatsch, and I Netuka are consultants for Abbott; L Damme is an employee of Abbott.
Ethical Statement: Study approval was obtained by the local ethics committee (study number: 6890MPG; DIMDI application number: 9567) and patient informed consent was obtained prior to treatment.
References
- Schmitto JD, Hanke JS, Rojas SV, et al. First implantation in man of a new magnetically levitated left ventricular assist device (HeartMate III). J Heart Lung Transplant 2015;34:858-60. [Crossref] [PubMed]
- Schmitto JD, Molitoris U, Haverich A, et al. Implantation of a centrifugal pump as a left ventricular assist device through a novel, minimized approach: upper hemisternotomy combined with anterolateral thoracotomy. J Thorac Cardiovasc Surg 2012;143:511-3. [Crossref] [PubMed]
- Netuka I, Sood P, Pya Y, et al. Fully magnetically levitated left ventricular assist system for treating advanced HF: a multicenter study. J Am Coll Cardiol 2015;66:2579-89. [Crossref] [PubMed]
- Hanke JS, Krabatsch T, Rojas SV, et al. In vitro evaluation of inflow cannula fixation techniques in left ventricular assist device surgery. Artif Organs 2017;41:272-5. [Crossref] [PubMed]
- Haberl T, Riebandt J, Mahr S, et al. Viennese approach to minimize the invasiveness of ventricular assist device implantation. Eur J Cardiothorac Surg 2014;46:991-6; discussion 996. [Crossref] [PubMed]
- Schmitto JD, Rojas SV, Hanke JS, et al. Minimally invasive left ventricular assist device explantation after cardiac recovery: surgical technical considerations. Artif Organs 2014;38:507-10. [Crossref] [PubMed]
- Schulz A, Stepanenko A, Krabatsch T.. HeartMate 3 implantation via left lateral thoracotomy with outflow graft anastomosis to the descending aorta. J Heart Lung Transplant 2016;35:690-2. [Crossref] [PubMed]
- Schmitto JD, Hanke JS, Rojas S, et al. Circulatory support exceeding five years with a continuous-flow left ventricular assist device for advanced heart failure patients. J Cardiothorac Surg 2015;10:107. [Crossref] [PubMed]
- Zimpfer D, Netuka I, Schmitto JD, et al. Multicentre clinical trial experience with the HeartMate 3 left ventricular assist device: 30-day outcomes. Eur J Cardiothorac Surg 2016;50:548-54. [Crossref] [PubMed]
- Krabatsch T, Netuka I, Schmitto JD, et al. Heartmate 3 fully magnetically levitated left ventricular assist device for the treatment of advanced heart failure -1 year results from the Ce mark trial. J Cardiothorac Surg 2017;12:23. [Crossref] [PubMed]
- Schmitto JD, Pya Y, Zimpfer D, et al. HeartMate 3 Fully Magnetically Levitated Left Ventricular Assist Device for the Treatment of Advanced Heart Failure - CE Mark Study 2-Year Results. J Heart Lung Transplant 2017;36:S66. [Crossref]
- Feldmann C, Chatterjee A, Hanke JS, et al. Novel centrifugal pump for heart failure patients: initial success and future challenges. J Thorac Dis 2017;9:1429-31. [Crossref] [PubMed]