Single nucleotide polymorphisms of casitas B-lineage lymphoma proto-oncogene-b predict outcomes of patients with advanced non-small cell lung cancer after first-line platinum based doublet chemotherapy
Introduction
The platinum-based doublet chemotherapy as the first-line treatment is a critical option for patients with advanced non-small cell lung cancer (NSCLC). However, the short term and long-term efficacies of chemotherapy have not been satisfying in clinical practice. Currently, the promising treatment for the advanced NSCLC is immunotherapy by modulating the host immune response, local immune effects and tumor microenvironment (1-3).
Accumulating results from published studies had illuminated the association of local immune status with chemotherapy or immunotherapy responses and clinical outcomes in various types of cancer (4-7). The capacity of lymphocytes, particularly the T cells that recognize tumor antigens, provides a potential actionable treatment by fighting against cancer (8). The proportions of various T cells (for examples, effecting T cells and regulating T cells) could be changed by chemotherapy (4,9). Therefore, the functions of T cells may be altered by chemotherapy, influencing on the tumor and thus patients’ prognosis.
The E3 ligase Cbl-b is one member of the Cbl family, which is vital in post-transcriptional protein modification by ubiquitination and also regulate several signaling pathways in various immune cells (10). Up-regulating Cbl-b in gastric cells can reduce the expression of p-glycoprotein (P-gp) and partially reverse chemotherapeutics resistance (11). Over expression of Cbl-b could enhance the efficacy of VP-16 (12) and promote drug sensitivity of anthracyclines (13). Conversely, many studies have suggested that deactivation of Cbl-b enhances CD8+ T cells’ capacity of killing tumor cells (14,15), reduces Foxo3a phosphorylation in regulatory T cells (16) and rejects cancer metastasis via natural killer cells (17). On the other hand, the dead tumor cells caused by chemotherapy could provide antigen to dendritic cells (DCs) and play vital role in anti-tumor immune (18). Furthermore, chemotherapeutics can decrease regulatory T cells and enhance anticancer immune responses in mice and humans (19,20). So Cbl-b was involved in modulation of chemotherapeutics sensitivity and anti-tumor immune responses. Previously, we reported that genotypes of rs2305035 in casitas B-lineage lymphoma proto-oncogene-b (CBLB) in 393 NSCLC Caucasian patients treated with chemoradiation predicted clinical outcomes and risk of radiation-induced pneumonitis (21). In the present study, we hypothesized that rs2305035 and other functional single nucleotide polymorphisms (SNPs) in CBLB might predict clinical outcomes in Chinese patients with advanced NSCLC treated with the platinum-based doublet chemotherapy as the first-line therapy.
Methods
Patient population
The present study initially included 200 patients with advanced NSCLC treated with the first-line platinum-based doublet chemotherapy at Henan Tumor Hospital (Zhengzhou, China) between October 2013 and April 2015, from whom DNA samples were available. The main chemotherapy regimens were pemetrexed plus platinum, gemcitabine plus platinum and taxanes plus platinum with 21 days per cycle. All patients were staged according to the 7th edition of the American Joint Committee on Cancer (AJCC) staging system. Patients who had completed 4 cycles of the first-line combined chemotherapy were included in the final analysis. Totally, there were 116 patients remained for the analysis of progression free survival (PFS) and 133 patients for the analysis of overall survival (OS) after excluding patients with missing follow-up information. According to RECIST 1.1, we evaluated tumor response by computed tomography (CT) at the time of every follow-up visit at the hospital.
Blood samples from all enrolled subjects were collected before treatment. Genomic DNA was extracted from peripheral blood leukocytes using a DNA extracting Kit (item No: SK8224, Sangon Biotech Co., Ltd., Shanghai, China) according to the manufacturer’s instructions. DNA purity and concentration were determined by spectrophotometric measurement of absorbance at 260 and 280 nm. Smoking index defined as the number of cigarettes smoked per day multiplied by the number of years smoked (22) was used to quantitate smoking. All subjects underwent complete evaluation of blood, differential white blood cell counts, coagulation function and D dimer level before any treatment. The neutrophil-lymphocyte ratio (NLR) was defined as the neutrophil counts divided by the lymphocyte counts, and the platelet-lymphocyte ratio (PLR) was defined as the platelet counts divided by the lymphocyte counts (23,24). NLR, PLR and D dimer were classified as patients’ characteristics that may have an effect on survival. The present study was approved by Henan Tumor Hospital’s institutional review board (No. 20120755) in compliance with Helsinki Declaration. And in the retrospective study, patients’ consents were waived.
SNP selection and genotyping
We searched the online SNP database (http://www.ncbi.nlm.nih.gov/projects/SNP; https://snpinfo.niehs.nih.gov/snpinfo/snpfunc.html; http://regulomedb.org/) and related literature to identify all potentially functional SNPs of CBLB with a minor allele frequency (MAF) ≥0.05 among subjects of Asian descent. We found six potentially functional SNPs (rs3772534, rs7649466, rs2305037, rs2305036, rs2305035 and rs1042852) with MAF ≥0.05 within the region of 20-kb up- and down-stream of the CBLB gene. Linkage disequilibrium (LD) analysis was used to optimize SNP selection to reduce redundant SNPs. Having removed SNPs in high LD (r2 >0.6), we finally selected three SNPs for genotyping: rs3772534 C>T; rs2305035 G>A; rs9657904 C>T.
The three SNPs in CBLB (rs3772534, rs2305035 and rs9657904) were genotyped using direct DNA sequencing method. The used sequencing primers including: rs2305035 (forward 5'-CTATTTGTTTAGGAGTCGGATGG-3', reverse 5'-CATCATCAAGGACTCCTCACG-3', amplifying a fragment of 192 bps); rs3772534 (forward 5'-GCCAATGCCTCTTGAAGC-3', reverse, 5'-GCTTTGCGTATTTCTTACCTTA-3', amplifying a fragment of 220 bps); rs9657904 (forward, 5'-ACAGTAGTTTTAAGAGCAGTGATCC-3', reverse, 5'-TGAATTGGAATTAAGGCAGG-3', amplifying a fragment of 204 bps). The PCR amplification system was performed in a total volume of 50 uL. The amplification conditions were used as follows: 94 °C for 10 min, followed by 35 cycles of 30 s at 94 °C, 30 s at 57 °C, and 30 s at 72 °C, and ending with a single 10-min extension step at 72 °C. In the genotyping experiments for the three SNPs, the digestion products were resolved by electrophoresis in 1.5% agarose gel.
Statistical analysis
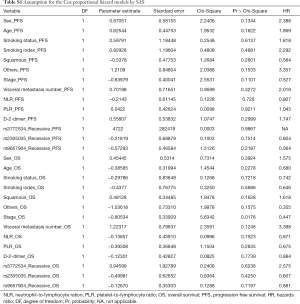
PFS was calculated from the date that treatment began to the first time of tumor progression, or date of death of any cause before tumor progression, or the last contact date. OS was calculated from the date of diagnosis until death or the date of last contact. The median survival time was estimated by using Kaplan-Meier method, and the survival curves were compared by using log-rank test. Multivariate Cox proportional hazards regression models were used to estimate the effect of each genotype [in terms of hazards ratios (HRs) and their corresponding 95% confidence intervals (CIs)] on PFS and OS with or without adjustment for selected factors by using the stepwise selection method in SAS (for PFS, two variables were adjusted: visceral metastasis number and PLR;for OS, the adjusted factor are smoking status and stage). The proportional hazard assumption was met for all clinical variables and SNPs (Table S1). All tests were two-sided, and a P value <0.05 was considered statistically significant. We also used the false-positive report probability (FPRP) to test for false positive associations. For all significant genetic effects observed in the present study, we calculated FPRP levels at 0.0001, 0.001, 0.01, 0.1, and 0.25. The HR was set close to 0.67 (protection) or 1.50 (risk) in the present study, and a probability value <0.2 was considered noteworthy. All data were analyzed with SAS 9.2 software (SAS Institute Inc., Cary, NC, USA), if not mentioned elsewhere.
Full table
Results
Patient characteristics
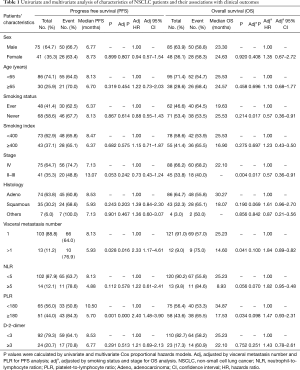
As shown in Table 1, in PFS analysis, there were 75 males and 41 females with a median age of 58 years (range, 23–82 years). There were 75 patients (64.7%) with stage IV, and 41 patients 35.3% had stage II–III (most of these patients received radiotherapy after or concurrent chemotherapy) diseases, all of whom received at least 4 cycles the first-line chemotherapy or induction chemotherapy. The adenocarcinoma accounted for 63.8% and squamous 30.2%, and most patients (88.8%) had one visceral metastasis (lung, bone or brain). Based on a previous study (24), 5 and 180 were defined by the threshold value for NLR and PLR, respectively, 92 patients had normal D-2-dimer, and 24 patients had elevated D-2-dimer. The overall median PFS for 116 patients was of 7.02 months.
Full table
Table 1 also shows the characteristics of 133 patients treated with the first-line chemotherapy for OS analysis. In this OS dataset, there were 85 males and 48 females, the median age was 59 years (range, 25–76 years), 88 patients (66.2%) had stage IV diseases, and 45 patients (33.8%) patients had stage II or III diseases. Other clinical characteristics were similar to the PFS dataset, and the overall median OS was of 24 months.
We found that patients with visceral metastasis number >1 and PLR ≥180 had a worse PFS in both univariate and multivariate Cox models. In the OS analysis, patients with never smoking or early stage had a longer OS in multivariate Cox models (Table 1). The adjustments for other factors were accomplished by a Cox stepwise model with all known clinical characteristics (visceral metastasis number and PLR for PFS, smoking status and stage for OS).
Associations of CBLB SNPs with PFS and OS
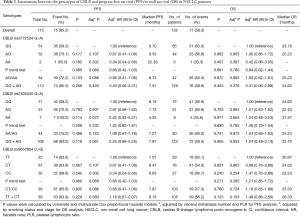
Associations of the three SNPs (rs3772534, rs2305035 and rs9657904) with PFS and OS were evaluated by using multivariate Cox models with adjustment for other selected factors (as shown in Table 2). However, we did not find any significant associations of either SNPs with PFS and OS (in a dominant genetic model for PFS, P=0.133, 0.498, and 0.935 for rs3772534, rs2305035, and rs9657904, respectively; in a dominant genetic model for OS, P=0.872, 0.741, and 0.780, respectively; in recessive model for PFS, P=0.266, 0.118, and 0.229, respectively; in a recessive genetic model for OS, P=0.403, 0.905, and 0.123, respectively).
Full table
Stratification analysis
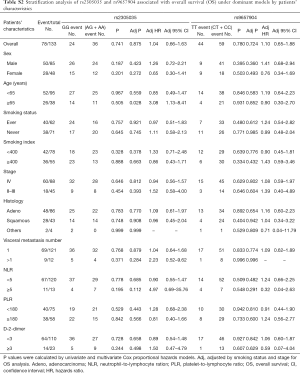
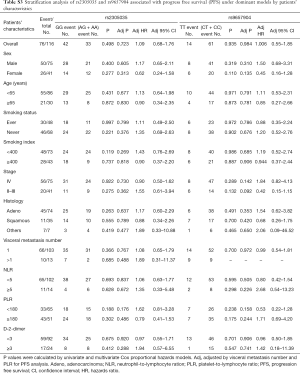
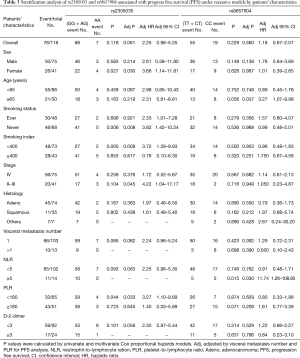
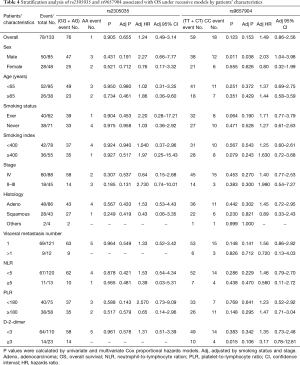
For assessing subgroup effects, we performed stratification analysis by clinical characteristics under both dominant and recessive genetic models. In the dominant genetic model, the results were shown in Tables S2 and S3. Only the rs2305035 AG + AA genotype was associated with OS in age ≥65 patients adjusted by smoking status and stage. While in the recessive genetic model, the rs2305035 AA genotype was more prominently associated with PFS in females, never smoking, smoking index <400 or PLR <180 (Table 3). Furthermore, the associations between the rs9657904 CC genotype and PFS by age ≥65 or NLR ≥5 were more remarkable (Table 3) (Figure 1A). In OS subgroup analysis, the rs9657904 CC genotype was significantly associated with OS in male patients (adjusted HR =2.03, 95% CI, 1.04–3.98, P=0.038) (Table 4) (Figure 1B).
Full table
Full table
Full table
Full table
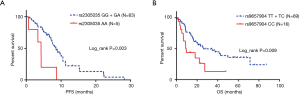
To provide functional evidence for the associations, we further evaluated the correlation between the potentially functional SNPs (rs9657904 and rs2305035) and CBLB mRNA expression levels using the public available mRNA expression data of 90 lymphoblastoid cell lines derived from eastern Asian population in HapMap (25). Consistent with the association results, the CC genotype of rs9657904 was shown to be associated with a relatively higher level of mRNA expression of CBLB, compared with the TT + TC genotypes (P=0.029) (Figure 2A). However, the genotype of rs2305035 was not associated with the CBLB mRNA expression (P=0.176) (Figure 2B).

FPRP analysis
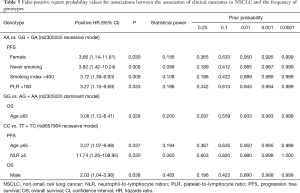
The FPRP values (26) were calculated at different prior probability levels for all significant findings (Table 5). As the assumption of prior probability was 0.25, the association of PFS with the AA genotype of rs2305035 was still noteworthy for patients with never smoking or smoking index <400 (FPRP =0.189, or 0.196). Meanwhile, the CC genotype of rs9657904 were also statistically associated with OS in male patients (FPRP =0.196).
Full table
Discussion
In the present study, we investigated the associations between potential functional SNPs in CBLB and the OS and PFS of patients with advanced NSCLC treated with the first-line combined chemotherapy. We found that the rs2305035 AA genotype was associated with a worse PFS in patients who were female, never smoking, had a smoking index <400 or PLR <400; and the rs9657904 CC genotype was associated with a worse PFS in patients with age ≥65 or NLR ≥5. In OS analysis, males’ patents with the rs9657904 CC genotype had a worse OS, compared with those males with rs9657904 TT + TC genotypes. The present study suggests that SNPs in CBLB might influence the PFS and OS of NSCLC patients with particular characteristics after the first-line combined chemotherapy in Chinese.
In our previously published study (21), we found that patients with AA/AG genotypes of rs2305035 had better clinical outcomes than GG carriers in Caucasian populations. However, such correlations were not found in the present study with Chinese patients. Such discrepancy might be due to ethnic difference, which had been reported to be one important confounder factor in association analysis (27,28). However, both studies suggested that SNPs in CBLB were associated with clinical outcomes in advanced NSCLC patients. In the present study, we had shown that the CC genotype of rs9657904 was associated with a higher level of CBLB mRNA and worse clinical outcomes in Chinese NSCLC patients with the first-line chemotherapy, which suggests that up-regulation of CBLB might influence the prognosis of NSCLC. Additional functional analyses are required to investigate the relationship between the prognosis of advanced NSCLC and Cbl-b expressions in tumor tissues.
Accumulating evidence has showed the prognosis of cancer patients and chemotherapy efficacy can be predicted by tumor immune micro-environment disparities, such as tumor-infiltrating lymphocytes (TILs) and T cells subsets. Adams et al. reported that stromal lymphocytic infiltration constituted a robust prognostic factor in triple-negative breast cancer; and higher stromal TIL scores were associated with better prognosis in multivariable analysis (29). Asano et al. found that tumor-infiltrating CD8 to FOXP3 lymphocyte ratio predicted treatment responses to neoadjuvant chemotherapy of aggressive breast cancer (4). Furthermore, maintenance of immune tolerance is a critical hallmark of the immune system. So far, CBLB appears to be a central player in balance activating and inhibitory inputs to immune cells (10). Several studies suggested silencing CBLB in vivo as a therapeutic strategy to target cancer (30,31). Besides, CBLB encodes a multifunctional adaptor protein in RING-family E3 ubiquitin ligase to negatively modulate the activation of T cell receptor (TCR) and B cell receptor (BCR) (32,33).
The identified SNP rs9657904 was reported to be associated with multiple sclerosis (MS), an auto-immune disease, in several genome wide association studies (GWAS) (34-36). In our findings, in male subgroup, the rs9657904 CC genotype of CBLB was associated with a worse OS, compared with the T genotypes, which was in consistence with studies of MS. In the previous studies (34), the allele T in rs9657904 (which is associated with an increased MS risk) was strongly associated with decreased expression of 234112_at, a probe that overlaps CBLB but is not part of its isoforms. From the above-mentioned evidence, it might be reasonable to hypothesize that decreased CBLB expression will enhance immune response by activating TCR and BCR. As the allele T of rs9657904 in the 5’ upstream of CBLB was associated with lower CBLB expression possibly, it would influence the immune system by enhancing anti-tumor immune response. Noticeably, downregulation of Cbl-b in gastric cancer cells and leukemia cells can reduce the sensitivity of chemotherapeutics by activation of PI3K/Akt pathway (11-13). While, it is unclear that the effect of Cbl-b on the sensitivity of chemotherapy in lung cancer cells. Nevertheless, down-regulation of Cbl-b can strengthen T cell anti-tumor immune response (14,15,31,37). PI3K was identified as a substrate for Cbl-b. The activation of Cbl-b (−/−) T cells can be suppressed by PI3K inhibitor (38). It is inconsistent that Cbl-b has different role in tumor cells and T cells without clarification. Thereby, the functions of Cbl-b research focusing on tumor cells, anti-tumor immune system and chemotherapeutics’ sensitivity should be conducted.
Similar to Cbl-b acting as a negative regulator in immune system, programmed death 1 (PD-1) on T cell surface and its ligand PD-L1 on tumor cell surface play a vital role in tumor immune escape. A recent study showed that overexpression of PD-L1 in NSCLC patients with surgery was associated with worse neoadjuvant chemotherapy efficacy. Furthermore, in vitro, the expression of PD-L1 was upregulated in cisplatin-resistance lung cancer cells by activation of the PI3K/Akt pathway; and knockdown of PD-L1 significantly reduced cisplatin resistances (39). Black et al. found that PD-1/PD-L1 axis can cause tumor cell resistance to chemotherapeutics. And inhibition of PD-1/PD-L1 by anti-PD-1 antibody strengthen doxorubicin to inhibit metastasis in vivo (40). According to the above evidences, immunotherapy targeting the immune response modulating genes might have synergistic effect with chemotherapy to eliminate tumor cells.
The present study has several limitations. First, this was a retrospective study and corresponding tumor tissues were not available for correlative studies of tumor-infiltrating immune cells. Secondly, it is still unclear about the underlying molecular mechanism of the association of rs2305035 and rs9657904 with clinical outcomes. Thirdly, the sample size of this study was relatively small, which might limit its power to detect other moderate effects and interaction effects on clinical outcomes, or lead to overestimation of the effect sizes of SNPs. Further larger and independent studies are warranted to verify our findings.
In conclusion, we found that the rs2305035 AA genotype and rs9657904 CC genotype of CBLB appeared to predict a shorter PFS in Chinese patients with some special characteristics and with advanced NSCLC treated with the first-line chemotherapy. In addition, the rs965790 CC genotype was associated with a worse OS in male patients. Finally, our findings suggest that CBLB has the potential to be a target of immunotherapy in future studies. It will be valuable and meaningful to clarify the comprehensive effects of CBLB on immune cells, tumor cells and chemotherapeutics’ sensitivity.
Acknowledgements
Funding: This work was supported by grant from National Science Foundation of China (No. 81672605).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The present study was approved by Henan Tumor Hospital’s institutional review board (No. 20120755) in compliance with Helsinki Declaration. And in the retrospective study, patients’ consents were waived.
References
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:2980-7. [Crossref] [PubMed]
- Asano Y, Kashiwagi S, Goto W, et al. Tumour-infiltrating CD8 to FOXP3 lymphocyte ratio in predicting treatment responses to neoadjuvant chemotherapy of aggressive breast cancer. Br J Surg 2016;103:845-54. [Crossref] [PubMed]
- Teng F, Meng X, Kong L, et al. Tumor-infiltrating lymphocytes, forkhead box P3, programmed death ligand-1, and cytotoxic T lymphocyte-associated antigen-4 expressions before and after neoadjuvant chemoradiation in rectal cancer. Transl Res 2015;166:721-32.e1. [Crossref] [PubMed]
- Diana A, Wang LM, D'Costa Z, et al. Prognostic value, localization and correlation of PD-1/PD-L1, CD8 and FOXP3 with the desmoplastic stroma in pancreatic ductal adenocarcinoma. Oncotarget 2016;7:40992-1004. [PubMed]
- Goff SL, Dudley ME, Citrin DE, et al. Randomized, Prospective Evaluation Comparing Intensity of Lymphodepletion Before Adoptive Transfer of Tumor-Infiltrating Lymphocytes for Patients With Metastatic Melanoma. J Clin Oncol 2016;34:2389-97. [Crossref] [PubMed]
- Noble F, Mellows T, McCormick Matthews LH, et al. Tumour infiltrating lymphocytes correlate with improved survival in patients with oesophageal adenocarcinoma. Cancer Immunol Immunother 2016;65:651-62. [Crossref] [PubMed]
- Romero I, Garrido F, Garcia-Lora AM. Metastases in immune-mediated dormancy: a new opportunity for targeting cancer. Cancer Res 2014;74:6750-7. [Crossref] [PubMed]
- Lutz-Nicoladoni C, Wolf D, Sopper S. Modulation of Immune Cell Functions by the E3 Ligase Cbl-b. Front Oncol 2015;5:58. [Crossref] [PubMed]
- Zhang Y, Qu X, Hu X, et al. Reversal of P-glycoprotein-mediated multi-drug resistance by the E3 ubiquitin ligase Cbl-b in human gastric adenocarcinoma cells. J Pathol 2009;218:248-55. [Crossref] [PubMed]
- Qu X, Li Y, Liu J, et al. Cbl-b promotes chemotherapy-induced apoptosis in rat basophilic leukemia cells by suppressing PI3K/Akt activation and enhancing MEK/ERK activation. Mol Cell Biochem 2010;340:107-14. [Crossref] [PubMed]
- Qu X, Zhang Y, Li Y, et al. Ubiquitin ligase Cbl-b sensitizes leukemia and gastric cancer cells to anthracyclines by activating the mitochondrial pathway and modulating Akt and ERK survival signals. FEBS Lett 2009;583:2255-62. [Crossref] [PubMed]
- Loeser S, Loser K, Bijker MS, et al. Spontaneous tumor rejection by cbl-b-deficient CD8+ T cells. J Exp Med 2007;204:879-91. [Crossref] [PubMed]
- Chiang JY, Jang IK, Hodes R, et al. Ablation of Cbl-b provides protection against transplanted and spontaneous tumors. J Clin Invest 2007;117:1029-36. [Crossref] [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Paolino M, Choidas A, Wallner S, et al. The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature 2014;507:508-12. [Crossref] [PubMed]
- Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007;13:1050-9. [Crossref] [PubMed]
- Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol 2004;34:336-44. [Crossref] [PubMed]
- Lutsiak ME, Semnani RT, De Pascalis R, et al. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood 2005;105:2862-8. [Crossref] [PubMed]
- Li P, Wang X, Liu Z, et al. Single Nucleotide Polymorphisms in CBLB, a Regulator of T-Cell Response, Predict Radiation Pneumonitis and Outcomes After Definitive Radiotherapy for Non-Small-Cell Lung Cancer. Clin Lung Cancer 2016;17:253-62.e5. [Crossref] [PubMed]
- Singh N, Aggarwal AN, Gupta D, et al. Quantified smoking status and non-small cell lung cancer stage at presentation: analysis of a North Indian cohort and a systematic review of literature. J Thorac Dis 2012;4:474-84. [PubMed]
- Proctor MJ, Morrison DS, Talwar D, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer 2011;47:2633-41. [Crossref] [PubMed]
- Neofytou K, Smyth EC, Giakoustidis A, et al. Elevated platelet to lymphocyte ratio predicts poor prognosis after hepatectomy for liver-only colorectal metastases, and it is superior to neutrophil to lymphocyte ratio as an adverse prognostic factor. Med Oncol 2014;31:239. [Crossref] [PubMed]
- Stranger BE, Forrest MS, Dunning M, et al. Relative impact of nucleotide and copy number variation on gene expression phenotypes. Science 2007;315:848-53. [Crossref] [PubMed]
- Wacholder S, Chanock S, Garcia-Closas M, et al. Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J Natl Cancer Inst 2004;96:434-42. [Crossref] [PubMed]
- Phipps-Green AJ, Hollis-Moffatt JE, Dalbeth N, et al. A strong role for the ABCG2 gene in susceptibility to gout in New Zealand Pacific Island and Caucasian, but not Maori, case and control sample sets. Hum Mol Genet 2010;19:4813-9. [Crossref] [PubMed]
- Tishkoff SA, Reed FA, Friedlaender FR, et al. The genetic structure and history of Africans and African Americans. Science 2009;324:1035-44. [Crossref] [PubMed]
- Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 2014;32:2959-66. [Crossref] [PubMed]
- Shi ZD, Li XF, Hao L, et al. Cbl-b gene silencing in splenic T lymphocytes as a therapeutic strategy to target the prostate cancer RM-1 cell tumors in immune competent mice. Eur Rev Med Pharmacol Sci 2014;18:3819-30. [PubMed]
- Zhou SK, Chen WH, Shi ZD, et al. Silencing the expression of Cbl-b enhances the immune activation of T lymphocytes against RM-1 prostate cancer cells in vitro. J Chin Med Assoc 2014;77:630-6. [Crossref] [PubMed]
- Schmitz ML. Activation of T cells: releasing the brakes by proteolytic elimination of Cbl-b. Sci Signal 2009;2:pe38. [Crossref] [PubMed]
- Qiao G, Lei M, Li Z, et al. Negative regulation of CD40-mediated B cell responses by E3 ubiquitin ligase Casitas-B-lineage lymphoma protein-B. J Immunol 2007;179:4473-9. [Crossref] [PubMed]
- Sanna S, Pitzalis M, Zoledziewska M, et al. Variants within the immunoregulatory CBLB gene are associated with multiple sclerosis. Nat Genet 2010;42:495-7. [Crossref] [PubMed]
- Corrado L, Bergamaschi L, Barizzone N, et al. Association of the CBLB gene with multiple sclerosis: new evidence from a replication study in an Italian population. J Med Genet 2011;48:210-1. [Crossref] [PubMed]
- Varadé J, Comabella M, Ortiz MA, et al. Replication study of 10 genes showing evidence for association with multiple sclerosis: validation of TMEM39A, IL12B and CBLB [correction of CLBL] genes. Mult Scler 2012;18:959-65. [Crossref] [PubMed]
- Stromnes IM, Blattman JN, Tan X, et al. Abrogating Cbl-b in effector CD8(+) T cells improves the efficacy of adoptive therapy of leukemia in mice. J Clin Invest 2010;120:3722-34. [Crossref] [PubMed]
- Fang D, Wang HY, Fang N, et al. Cbl-b, a RING-type E3 ubiquitin ligase, targets phosphatidylinositol 3-kinase for ubiquitination in T cells. J Biol Chem 2001;276:4872-8. [Crossref] [PubMed]
- Zhang P, Ma Y, Lv C, et al. Upregulation of programmed cell death ligand 1 promotes resistance response in non-small-cell lung cancer patients treated with neo-adjuvant chemotherapy. Cancer Sci 2016;107:1563-71. [Crossref] [PubMed]
- Black M, Barsoum IB, Truesdell P, et al. Activation of the PD-1/PD-L1 immune checkpoint confers tumor cell chemoresistance associated with increased metastasis. Oncotarget 2016;7:10557-67. [Crossref] [PubMed]


