Single agent anti PD-1 inhibitors in esophageal cancer—a first step in a new therapeutic direction
Esophageal squamous cell carcinomas and adenocarcinomas have proven to be inherently resistant to systemic treatments as a result of histological, molecular and etiological heterogeneity, with limited responses seen after first line therapy. The promise of immunotherapy in esophagogastric cancer has been suggested for a long time due to the recognized link between infection, chronic inflammation, and malignancy. The emerging clinical trial data is somewhat confusing but it appears that anti programmed death-1 (PD-1)/programmed death ligand-1 (PD-L1) monoclonal antibodies do demonstrate some efficacy in a minority of gastroesophageal cancer patients with metastatic disease but with less activity when compared to melanoma or lung cancer.
Recent phase III data from the Keynote 059 and Attraction 2 studies demonstrate response rates of approximately 12% in a population of heavily pretreated patients and there was an overall survival benefit in the Attraction 2 trial (1,2). It is important to highlight however, that in both these studies, single agent PD-1 inhibitors (pembrolizumab or nivolumab) were compared to placebo rather than chemotherapy. The Keynote 059 study was a large (n=259 patients) multi-cohort phase II open label study which assessed the efficacy of pembrolizumab in patients with advanced gastric or gastro-esophageal junction (GEJ) cancers who had been previously treated with two or more previous lines of chemotherapy (1). The median duration of follow-up was 5.4 months; objective response rates (ORR) of 11.2% were observed with a median duration of response of 8.1 months. Among those being treated in the third line setting, the ORR was 14.9%, compared to 7.2% in the fourth line. In those with PD-L1 positive tumors (n=148) (defined as PD-L1 ≥1% in tumor or stromal cells by IHC), ORR was noted to be 15.5% compared to 5.5% in PD-L1 negative tumors, and was higher in those treated in the third line setting (ORR 21.3%) (1). As a result of this study, in Sept 2017 the FDA approved the use of pembrolizumab in the United States in the 3rd line metastatic setting for gastric or gastroesophageal junction adenocarcinomas whose tumors express PD-L1.
Similarly, the ONO-4538-12 (ATTRACTION-2) was a multicenter double-blind phase III study which randomized Asian patients (n=493, Japan, South Korea and Taiwan) with unresectable advanced or recurrent gastric or EGJ cancer treated with two or more prior lines of therapy to nivolumab or placebo (2). The median OS was 5.3 vs. 4.1 months in the nivolumab and placebo groups respectively, with 12-month OS rates of 27% and 11% (HR =0.63; P<0.0001) in the nivolumab and placebo groups respectively. The median PFS was 1.6 months with nivolumab compared to 1.45 months for placebo (HR =0.60; P<0.0001). The overall response rate was 11% with nivolumab versus 0% for placebo, with a median duration of response of 9.53 months with nivolumab (2). Based on this study, in September 2017, the Japanese Ministry of Health, Labor and Welfare approved nivolumab for the treatment of unresectable advanced or recurrent gastric cancer which progressed after chemotherapy.
Unlike these placebo-controlled studies, recent press releases from two large phase III studies have somewhat dampened the enthusiasm for single agent PD-1/PD-L1 inhibitors in unselected patients with metastatic esophageal and gastric cancer, when compared to chemotherapy. Keynote 061 was a randomized open-label phase III study investigating pembrolizumab monotherapy versus paclitaxel in patients with advanced gastric or GEJ adenocarcinomas who had previously progressed on combination platinum 5-fluorouracil chemotherapy (3). This study did not meet its primary endpoint of improved OS or PFS in patients with PD-L1 expressing tumors treated with pembrolizumab, so the pre-planned analysis of the overall population (PD-L1 positive and PD-L1 negative) was not carried out (4). Similarly, the Javelin Gastric 300 study was an open-label phase III study comparing the PD-L1 inhibitor avelumab plus best supportive care (BSC) versus BSC with or without paclitaxel or irinotecan chemotherapy as third line treatment for patients with unresectable recurrent or metastatic gastric or GEJ adenocarcinoma (5). Like the Keynote 061 study, the Javelin Gastric 300 study did not meet its primary endpoint of improved OS with single agent avelumab compared to physicians’ choice chemotherapy (6). We await the formal presentations of the results of both of these studies.
What do these four studies tell us? Looking at the Kaplan Meier curves for survival there is certainly a tail in the curves which indicates that there is a population of patients who do derive benefit from single agent PD-1 inhibition in the metastatic setting. This population does however appear to be smaller when compared with the responses seen in other tumor types such as melanoma and lung cancer. We clearly need a much greater immunologic/molecular understanding of biological phenomena that lead to the development and progression of esophageal cancer and a comprehensive analysis of the immune microenvironment not just in the metastatic setting but at various stages throughout a cancers lifespan. If single agent chemotherapy is a better strategy than single agent PD-1 inhibitors for the majority of patients then we clearly need to look at IO-IO combination strategies or combining PD-1 inhibitors with chemotherapy. These studies are ongoing and preliminary results are promising but the science needs to guide our clinical trial designs.
The Cancer Genome Atlas Research Network recently performed a comprehensive molecular analysis of 164 esophageal tumors, 359 gastric adenocarcinomas and 36 additional adenocarcinomas at the GEJ (7). Beyond the known histopathological and epidemiologic distinctions, this work has identified molecular features that can further help differentiate esophageal squamous cell carcinomas from esophageal adenocarcinomas and may explain differential responses to immunotherapeutics. In fact, it appears that esophageal squamous cell carcinomas (ESCC) resemble head and neck squamous carcinomas more than esophageal adenocarcinomas (EAC). Squamous cell carcinomas showed frequent genomic amplifications of CCND1 and SOX2 and/or TP63, whereas ERBB2, VEGFA and GATA4 and GATA6 were more commonly amplified in adenocarcinomas. Esophageal adenocarcinomas resemble the chromosomally unstable variant of gastric adenocarcinoma, suggesting that these cancers could be considered a single disease and that esophageal squamous cell carcinomas should be treated as a separate entity. Until recently our knowledge of the immune microenvironment of ESSC has similarly been limited, and a much greater understanding of the underlying immune milieu is required if we are to use checkpoint inhibitors differently in EAC and ESCC. In 2018, we know that PD-L1 upregulation occurs in approximately 40% of gastro-esophageal cancers. However, unlike other solid tumors there is little PD-L1 expressed on the cancer cells but rather expression occurs predominantly on infiltrating myeloid cells at the invasive margin (8-10).
Additionally, subsets of gastro-esophageal cancer with different immune signatures, most notably Epstein-Barr virus (EBV) positive and mismatch repair deficient tumors have been identified (11). In the 10% of gastric cancers that are EBV positive, approximately 50% and 94% PD-L1+ staining is seen on tumor cells and immune cells, respectively. Mismatch repair deficiency or microsatellite instability (MSI) also impacts PD-L1+ status with tumor and immune cells staining positive in 33% and 45% of cases, respectively with both subtypes having PD-L1+ immune cells with tumor infiltrating patterns (11). Mismatch repair is however a gastric only phenomenon with less than 0.5% of GEJ tumors demonstrating deficiency in this pathway and even less in esophageal squamous cell carcinoma (12). Recent studies have highlighted the relevance of tumor neoantigen landscape and how this may impact sensitivity to checkpoint inhibition. Tumors with both a high clonal neoantigen burden and low neoantigen intratumoral heterogeneity have been associated with an inflamed tumor microenvironment enriched with activated effector T cells, greater PD-L1 expression, and significantly longer progression free survival (13).
In the article prompting this editorial, Kudo et al. performed an open label phase II study, assessing the safety and activity of nivolumab in patients with treatment refractory squamous cell esophageal cancer (14). Although the investigators allowed esophageal squamous cell carcinomas, adeno-squamous carcinomas or adenocarcinomas to be enrolled in this study, all 65 cases were found to be squamous cell carcinomas. The primary tumor may have been previously resected or unresected, but was limited to the upper two-thirds of the esophagus, as is often the case in Japan, and with squamous histologies. Patients must have been previously treated with, and found to be refractory, or intolerant, to fluoropyrimidine-based, platinum-based or taxane-based chemotherapy. One notable exclusion criterion was locally advanced disease with tumor invasion into surrounding structures such as the aorta or respiratory tract. The primary endpoint of this study was objective response, defined as either CR or PR by RECIST 1.1.
The median age in this study was 62 (range, 49–80) with a male predominance (83%). Ninety-three percent (93%) were current or former smokers, and 96% were current or former alcohol users. Sixty-eight percent of patients had been treated with prior surgery, and a further 68% were treated with prior radiotherapy. All patients had been treated with prior chemotherapy, as per the inclusion criteria: 32% with ≤2 prior regimens, 37% with 3 prior regimens and 31% with ≥4 regimens, representing a heavily pre-treated population. Patients were treated with a median of 3 cycles of nivolumab with a median follow-up of 10.8 months. Eight-five percent (85%) of patients were found to have adverse events: 26% grade 3–4 and 17% serious grade 3–4 events. Treatment related adverse events were reported in 60% of patients, with 17% having grade 3 or higher events. There were no reported treatment related deaths.
The number of patients having an objective response ranged from 17–22% based on central and investigator assessments. Disease control was seen in 42–53%. The median OS was 10.8 months, with PFS of 1.5 and 2.3 months by central and investigator assessment respectively. The median time to progression was 2.8 months. The median time to response was noted to be 1.5 months but interestingly the median duration of response was not reached, reflecting the sustained responses that are well described with the use of immune checkpoint inhibitors. These results are comparable to other open label studies namely Checkpoint 032 (15) and ATTRACTION-1 (16,17) study showing response rates of 12% and 17% respectively with nivolumab.
The study shows that nivolumab has activity in heavily pre-treated patients with advanced esophageal squamous cell carcinoma that are refractory or intolerant to standard of care chemotherapy regimens. Despite 67% of patients being treated with 3 or more prior lines of therapy, nivolumab was well tolerated in this cohort of patients, with an acceptable toxicity profile. The authors acknowledge that the RECIST criteria is inadequate to assess tumor response to immunotherapy agents, as once disease progression is recorded, further evaluation is not performed with RECIST. For example, Hoos et al. (18) reported that measurable anti-tumor activity might be longer for immunotherapy agents compared to conventional cytotoxic drugs, responses to immunotherapies may occur after initial progression of disease and therefore treatment should not be stopped too prematurely, and that durable stable disease may represent significant antitumor activity. Therefore, in this study by Kudo et al. (14), immune-related objective response, immune-related PFS and immune-related best objective response were also evaluated.
Finally, the authors conclude that these results, while thought provoking, may not be generalizable, as a very specific cohort of Japanese squamous cell esophageal carcinomas were studied. Genetic profiling of the tumors was not carried out, nor was biomarker assessment so it is unclear if there was a subset of tumors with preferential responses to nivolumab. However, a randomized phase III study of nivolumab compared to taxane monotherapy in locally advanced or recurrent esophageal cancer is ongoing, with predetermined biomarker evaluation (NCT02569242). Both the current study by Kudo et al. (14) and the subsequent ATTRACTION-2 study (2) have suggested that the PD-1 inhibitor nivolumab has a therapeutic benefit in both squamous cell esophageal carcinomas and gastric or EGJ cancers among Asian patients. Further studies are needed before these results can be deemed relevant in the non-Asian population.
It is our opinion that single agent PD-1 inhibitors will not be an effective treatment strategy for the majority of patients with heavily pre-treated metastatic gastric, esophageal or GEJ cancers. This strategy certainly represents a therapeutic step forward but we still have a long way to go if all patients are to derive benefit. There is emerging evidence that combinations of immune checkpoint inhibitors or immune checkpoint inhibitors plus chemotherapy are more likely to yield higher response rates, and ideally prolonged durations of response. In the CheckMate 032 study (15), nivolumab plus ipilimumab was superior to nivolumab alone, with ORR of 24% and 12% respectively. Preliminary data from the ATTRACTION-4 study (19), albeit with small patient numbers, have shown ORR ~70% when nivolumab was combined with either SOX (67%) or CapeOx (71%) chemotherapy in the first-line treatment of unresectable advanced or recurrent gastric or GEJ cancers. Similarly, cohort 2 of the Keynote 059 study (20) assessing the combination of pembrolizumab plus the combination of cisplatin and either 5FU or capecitabine, showed ORR of 60%, increasing to 69% in PD-L1 positive patients. Although formal publications of these studies are awaited, these response rates, if they a reproducible in the larger phase III trials of chemo plus IO, would be the highest we have ever seen in the first line metastatic setting.
Several trials are currently studying the use of combination immune checkpoint inhibitors (see Tables 1,2). The ongoing Checkmate-649 study (21) is assessing dual immune checkpoint inhibitors nivolumab plus ipilimumab versus combination chemotherapy (XELOX or FOLFOX) versus FOLFOX plus nivolumab in the first line setting of metastatic gastric or GEJ cancers (NCT02872116). The BMS Fraction-study (Fast Real-Time Assessment of Combination Targeted ImmunoOncology) is a basket study assessing multiple IO-IO combinations including the combination of nivolumab plus ipilimumab, nivolumab plus relatlimab (LAG-3 inhibitor) or nivolumab plus BMS-986205 (IDO inhibitor) in advanced gastric/GEJ cancer (NCT 02935634). Similarly, the Roche-Genentech Morpheus study is an open-label umbrella study evaluating multiple immunotherapy based treatment combinations in patients with locally advanced, unresectable or metastatic gastric or GEJ cancers (NCT 03281369).

Full table
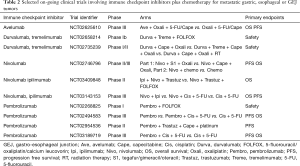
Full table
In the future, it is likely that single agent PD-1 inhibition in esophageal carcinomas will be replaced by doublet or even triplet IO-IO combinations or by combining IO with chemotherapy or indeed radiotherapy. This study by Kudo et al. (14), is however, an important step on the correct path as we look to bridge the gap from promising concept and turn immunotherapy into a therapeutic reality for all patients with esophageal cancer rather than the select few.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. R. Kelly has received research funding from BMS, Astrazeneca and Eli Lilly. Dr. R. Kelly is an advisory board member for BMS, Eli Lilly, Astellas, GritStone Oncology, EMD Serono, Novartis. Dr. E. Walsh has no conflicts of interest to declare.
References
- Fuchs CS, Doi T, Jang RW, et al. KEYNOTE-059 cohort 1: Efficacy and safety of pembrolizumab (pembro) monotherapy in patients with previously treated advanced gastric cancer. J Clin Oncol 2017;35:abstr 4003.
- Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:2461-71. [Crossref] [PubMed]
- Ohtsu A, Tabernero J, Bang YJ, et al. Pembrolizumab versus paclitaxel as second-line therapy for advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma: Phase 3 KEYNOTE-061 study. J Clin Oncol 2016. [Epub ahead of print]. [Crossref]
- Merck Provides Update on KEYNOTE-061, a Phase 3 Study of KEYTRUDA® (pembrolizumab) in Previously Treated Patients with Gastric or Gastroesophageal Junction Adenocarcinoma [press release]. Available online: http://investors.merck.com/news/press-release-details/2017/Merck-Provides-Update-on-KEYNOTE-061-a-Phase-3-Study-of-KEYTRUDA-pembrolizumab-in-Previously-Treated-Patients-with-Gastric-or-Gastroesophageal-Junction-Adenocarcinoma/default.aspx
- Bang YJ, Wyrwicz L, Park YI, et al. Avelumab (MSB0010718C; anti-PD-L1) + best supportive care (BSC) vs BSC ± chemotherapy as third-line treatment for patients with unresectable, recurrent, or metastatic gastric cancer: The phase 3 JAVELIN Gastric 300 trial. J Clin Oncol 2016. [Epub ahead of print].
- Merck KGaA, Darmstadt, Germany, and pfizer provide update on phase III javelin gastric 300 study in patients with Pre-treated advanced gastric cancer [press release]. Available online: https://www.pfizer.com/news/press-release/press-release-detail/merck_kgaa_darmstadt_germany_and_pfizer_provide_update_on_phase_iii_javelin_gastric_300_study_in_patients_with_pre_treated_advanced_gastric_cancer
- Cancer Genome Atlas Research N, Analysis Working Group: Asan U, Agency BCC, et al. Integrated genomic characterization of oesophageal carcinoma. Nature 2017;541:169-75. [Crossref] [PubMed]
- Thompson ED, Zahurak M, Murphy A, et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut 2017;66:794-801. [Crossref] [PubMed]
- Kelly RJ, Zaidi AH, Smith MA, et al. The Dynamic and Transient Immune Microenvironment in Locally Advanced Esophageal Adenocarcinoma Post Chemoradiation. Ann Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol 2016;17:717-26. [Crossref] [PubMed]
- Derks S, Liao X, Chiaravalli AM, et al. Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget 2016;7:32925-32. [Crossref] [PubMed]
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409-13. [Crossref] [PubMed]
- McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016;351:1463-9. [Crossref] [PubMed]
- Kudo T, Hamamoto Y, Kato K, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol 2017;18:631-9. [Crossref] [PubMed]
- Janjigian YY, Ott PA, Calvo E, et al. Nivolumab ± ipilimumab in pts with advanced (adv)/metastatic chemotherapy-refractory (CTx-R) gastric (G), esophageal (E), or gastroesophageal junction (GEJ) cancer: CheckMate 032 study. J Clin Oncol 2017;35:abstr 4014.
- Kojima T, Hara H, Yamaguchi K, et al. Phase II study of nivolumab (ONO-4538/BMS-936558) in patients with esophageal cancer: Preliminary report of overall survival. J Clin Oncol 2016. [Epub ahead of print]. [Crossref]
- Kitagawa Y, Doki Y, Kato K, et al. Two year survival and safety update for esophageal squamous cell carcinoma treated with nivolumab (ATTRACTION-01/ONO-4538-07). Ann Oncol 2017;28:v209-68. [Crossref]
- Hoos A, Parmiani G, Hege K, et al. A clinical development paradigm for cancer vaccines and related biologics. J Immunother 2007;30:1-15. [Crossref] [PubMed]
- Chen L, Kang Y, Tanimoto M, et al. ATTRACTION-04 (ONO-4538-37): A Randomized, Multicenter, Phase 2/3 Study of Nivolumab (Nivo) Plus chemotherapy in Patients (Pts) with Previously Untreated Advanced or Recurrent Gastric or Gastroesophageal Junction Cancer. Ann Oncol 2017;28:mdx369.159.
- Bang YJ, Muro K, Fuchs CS, et al. KEYNOTE-059 cohort 2: Safety and efficacy of pembrolizumab (pembro) plus 5-fluorouracil (5-FU) and cisplatin for first-line (1L) treatment of advanced gastric cancer. J Clin Oncol 2017;35:abstr 4012.
- Janjigian Y, Adenis A, Aucoin J, et al. Checkmate 649: A randomized, multicenter, open-label, phase 3 study of nivolumab (Nivo) plus ipilimumab (Ipi) versus oxaliplatin plus fluoropyrimidine in patients (Pts) with previously untreated advanced or metastatic gastric (G) or gastroesophageal junction (GEJ) cancer. J Clin Oncol 2017. [Epub ahead of print]. [Crossref]


