Effect of low molecular weight heparin on venous thromboembolism disease in thoracotomy patients with cancer
Introduction
Deep vein thrombosis (DVT) and pulmonary thromboembolism (PE) are both clinical expressions of venous thromboembolism (VTE), and are major surgery-related complications. These two almost coexist, and 82% of venography-proven PE have ultrasound evidence of DVT (1). Furthermore, approximately one-third of 150,000–200,000 VTE-related deaths per year in the United States occurred following surgery (2). Moreover, PE is associated with 0.2–10% of hospital deaths in Western countries (3-5). In Asia, VTE has historically been perceived to be a rare disorder. However, new evidence showed VTE was not rare in post operation patients in Asian population (6). The American College of Chest Physicians (ACCP), the National Comprehensive Cancer Network (NCCN), and the European Society of Cardiology (ESC) have also published guidelines for VTE prevention (7-9). However, there are few published data on VTE in patients following thoracotomy (10). The present study was designed to prospectively and randomly observe post-thoracotomy cancer patients (including lung cancer and esophagus cancer) to determine whether there was any difference between low molecular weight heparin (LMWH) combined mechanical prophylaxis and mechanical prophylaxis alone in the VTE prevention.
Methods
A prospective, randomized controlled design was used to observe post-thoracotomy cancer patients (including lung cancer and esophagus cancer) to determine whether there are any differences between LMWH combined mechanical prophylaxis and mechanical prophylaxis alone in preventing VTE. At the same time, the possible complications of LMWH were observed such as bleeding and heparin-induced thrombocytopenia (HIT).
Patients
The present study was conducted by the Department of Thoracic Surgery and Intensive Care Unit (ICU) at Peking University Cancer Hospital from October 2010 to September 2011 in Beijing, China. The protocol was approved by the Institutional Review Board of Peking University Cancer Hospital & Institute on June 14, 2010 (No. 2010061406). Written informed consent was obtained from all patients. Inclusion criteria: (I) age between 18 and 80 years old; (II) pathological diagnosis of malignant tumors; (III) scheduled for thoracotomy under general anesthesia; (IV) all anticoagulant treatment stopped for at least 7 days preoperatively; (V) normal coagulation function or mild coagulation dysfunction (PT <3 seconds above the upper limit, APTT <10 seconds above the upper limit); (VI) preoperative VTE excluded by computed tomographic pulmonary angiography (CTPA) or extremity venous ultrasound. Exclusion criteria: (I) postoperative therapeutic anticoagulants requirement such as for heart valve replacement; (II) active bleeding or transfusion RBC was >2 units within 24 hours; Active bleeding was defined as bloody chest drainage more than 200 mL per hour for 5 hours; Or patients had symptom of hypovolemic shock (III) tumor metastasis; (IV) platelet (PLT) count of <10×109/L.
Outcomes
The primary end points were incidence of pulmonary embolism (PE), DVT, and the PE severity index (PESI) of PE patients. CTPA and extremity venous Doppler ultrasound were performed in all patients on postoperative day (POD) 7. Simultaneously, patients diagnosed with PE by radiologist through CTPA were assessed using the PESI.
The secondary end points were hemoglobin (HGB), PLT, D-dimer (mcg/L), the PO2/FiO2 ratio (P/F) at POD7 and the chest drainage time (CDT) and the length of stay (LOS) in hospital after operation. PO2 were measured by arterial blood gas. FiO2 (fraction of inspiration oxygen) were recorded by nurses when arterial blood gas was measured. HGB, PLT, D-dimer, and P/F were measured in all patients on POD1 and POD7. CDT meant keeping chest drainage tubes time from operation to remove all chest drainage tubes. CDT and LOS were recorded in all patients postoperatively and were compared between the two groups.
Intervention
Eligible patients were randomly divided into two groups using a random number table: groups A and B. In group A, patients were given intermittent pneumatic compression (IPC) for 30 minutes, twice a day, and elastic stockings (ES) postoperatively, as a standard protocol in our department. Additionally, at 24 hours post-operation, patients in group A were subcutaneously injected with LMWH calcium (nadroparin calcium; GlaxoSmithKline, China) at 0.3 mL/2,850 anti-Xa IU, Qd for 7 days. In group B, patients were only given postoperative IPC and ES. All other treatments were the same in these two groups, including blood glucose control and fluid treatment; and if necessary, antibiotics and organ functional support were provided. Although the ICU physicians and surgeons knew the groupings of the patients, they could not change the treatment due to the groupings.
CTPA were analyzed and recorded by one radiologist who was blinded to the treatment group and outcomes. Patients diagnosed with VTE were managed according to ACCP therapy guidelines (11).
Statistical analysis
Power calculation
The sample size was calculated based on the differences in incidence of VTE between these two groups. In a previous study (12), postoperative thromboembolism occurred in 19% of patients after thoracic surgery. It was expected that the incidence of VTE in group A could be further reduced 50% with prophylaxis. The result of the calculation indicated that 236 patients were required to achieve a power of 0.80 with a two-tailed alpha of 0.05. In our hospital, more than 400 thoracotomies are performed every year. Hence, 75% of these cases were expected to be enrolled into the study. That is, there would be approximately 300 cases in one year. Therefore, it was anticipated that this study would be completed within 1 year.
Data were analyzed using Statistical Package for the Social Sciences (SPSS, version 16.0, IBM). Qualitative variables were expressed as number and percentage and were compared using Chi-square test. Furthermore, quantitative variables were expressed as mean ± standard deviation, and were compared using Student’s t-test. The difference between variables was considered statistically significant when the P value was ≤0.05.
Results
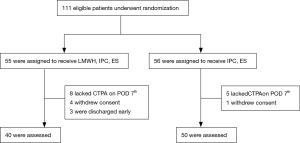
A total of 111 eligible inpatients were enrolled into the present study from October 2010 to September 2011. These patients were randomly divided into two groups: group A (n=55), and group B (n=56). Among them, 13 patients did not undergo CTPA on POD7, five patients withdrew their consents and three patients were lost to follow up due to early discharge. Finally, 40 patients in group A and 50 patients in group B were included into the study (Figure 1).
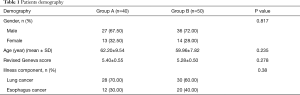
There were no significant differences in gender, age and primary disease (lung cancer-to-esophagus cancer ratio) between these two groups. Furthermore, the revised Geneva score was 5.40±0.55 and 5.28±0.50, respectively. This meant that both groups were at moderate risk for PE (P=0.278) (Table 1) (13).
Full table
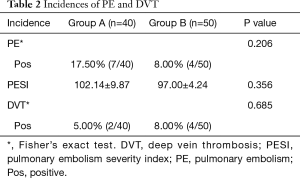
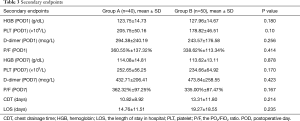
At POD7, the difference in the incidence of PE and DVT between these two groups was not statistically significant. Seven (17.50%) and 4 (8.00%) patients were diagnosed with PE in groups A and B, respectively (P=0.206); while 2 (5.00%) and 4 (8.00%) patients were diagnosed with DVT in groups A and B, respectively (P=0.685). There was no significant difference in PESI in PE patients between these two groups (102.14±9.87 and 97.00±4.24, respectively; P=0.356; Table 2). Furthermore, the differences in HGB (g/dL), PLT (×109/L), D-dimer (mcg/L), and P/F (%) at POD1 or POD7, as well as in CDT (days) or LOS (days), were not statistically significant between these two groups (Table 3). So nadroparin calcium did not increase the risk of bleeding or HIT. Merely one patient in group B was transfused with red blood cells due to bleeding caused by failure of surgical hemostasis, which was resolved by reoperation.
Full table
Full table
Discussion
In the present study, we compared the effect of LMWH combined IPC and ES with IPC and ES alone in cancer patients at post-thoracotomy and found no significant difference in the incidence of VTE, or PE and DVT individually.
Cancer patients undergoing thoracic surgery have been generally considered to be moderate risk for VTE (7). The high risk for VTE in this population, other than cancer itself (10,14), can be attributed to the fact that these patients often have additional VTE risk factors (13-15), including advanced age, malignancy, obesity, smoking, and prolonged immobilization, especially if mechanically ventilated. In the latest guidelines, ACCP recommended: For thoracic surgery patients at high risk for VTE who are not at high risk for perioperative bleeding, we suggest LDUH (grade 1B) or LMWH (grade 1B) over no prophylaxis. In addition, we suggest that mechanical prophylaxis with ES or IPC should be added to pharmacologic prophylaxis (grade 2C) (7). The European Society of Medical Oncology recommends that patients who underwent thoracotomy or thoracoscopy that lasted for more than 30 minutes should be considered for LMWH (16).
The present study did not reveal that LMWH combined with mechanical prophylaxis (IPC and ES) had better results either in the incidence of VTE or in hospital LOS, compared with mechanical prophylaxis alone. Our data are in accordance with some of the previous studies. Junichi et al. (17) compared patients who only received IPC and ES to patients who received subcutaneous unfractionated heparin (UFH) after pulmonary operation, in addition to IPC and ES, within a 4-year period and found no significant difference. In the study conducted by Kakkar et al. (18), it sought to determine whether LMWH can reduce the rate of death from any cause. A total of 8,307 patients were randomly divided into two groups: the enoxaparin group received enoxaparin plus ES and IPC (4,171 patients), while the placebo group received placebo plus ES and IPC (4,136 patients). The all-cause mortality rate at day 30 was 4.9% in the enoxaparin group and 4.8% in the placebo group (P=0.83). It should be noted that in all of these studies, including this study, patients in the matched group were only treated with mechanical prophylaxis (IPC + ES), but without any prophylaxis. Mechanical prophylaxis has been confirmed to prevent patients from VTE. In the study conducted by Nagahiro (19), 706 patients who underwent general thoracic surgery were assessed. Patients who received prophylactic IPC had a lower incidence of PE compared with patients who did not receive any prophylactic treatment. Furthermore, there was a statistical correlation between the occurrence of PE and the application of IPC (χ2-test, P=0.006). In the study of Eppsteiner et al. (20), conducted a meta-analysis of 16 randomized controlled trials on mechanical compression vs. subcutaneous heparin and found no significant difference in thromboembolic outcomes (DVT and PE) for postsurgical and post-trauma patients.
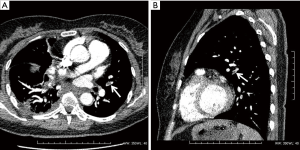
In this study, the incidence of DVT was similar with previous studies, but the incidence of PE was slightly higher than some other studies. We consider that the main reason was that in the present study, patients were diagnosed with PE by CTPA regardless of the symptoms, while in other studies, patients diagnosed with PE were symptomatic. In fact, none of the PE patients in the present study was symptomatic. Thus, the incidence of symptomatic PE in the present study should be 0%. CTPA revealed the presence of thrombus in segmental arteries and more distal arteries in most of the PE patients (the arrows in Figure 2A and 2B point to the thromboembolism in the pulmonary artery). Therefore, the present study also supplies evidence of the real incidence of PE at post-thoracotomy, including symptomatic and asymptomatic patients. Additional investigations should be conducted to investigate the effect of LMWH use on VTE rate.
Indeed, the present study has certain limitations. Firstly, patients received IPC for 30 minutes, twice daily which was shorter than other studies. This is a standard protocol in our department, and our study validated the beneficial effect on preventing VTE. Secondly, patients included into the present study came from a single center, and something related to not achieving the appropriate number of enrolled patients based on our power analysis.
Conclusions
LMWH combined with mechanical prophylaxis (IPC and ES) was not better than mechanical prophylaxis alone in preventing VTE (including PE and DVT) in thoracotomy cancer patients who were at moderate risk of PE, and it did not decrease CDT and LOS. Furthermore, LMWH did not decrease the HGB and PLT levels of patients and no bleeding events in LMWH group. It is possible that it would not increase the risk of bleeding or HIT.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The protocol was approved by the Institutional Review Board of Peking University Cancer Hospital & Institute on June 14, 2010 (No. 2010061406). Written informed consent was obtained from all patients.
References
- Girard P, Musset D, Parent F, et al. High prevalence of detectable deep venous thrombosis in patients with acute pulmonary embolism. Chest 1999;116:903-8. [Crossref] [PubMed]
- Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979-1998: an analysis using multiple-cause mortality data. Arch Intern Med 2003;163:1711-7. [Crossref] [PubMed]
- Baglin TP, White K, Charles A. Fatal pulmonary embolism in hospitalized medical patients. J Clin Pathol 1997;50:609-10. [Crossref] [PubMed]
- Sandler DA, Martin JF. Autopsy proven pulmonary embolism in hospital patients: are we detecting enough deep vein thrombosis? J R Soc Med 1989;82:203-5. [Crossref] [PubMed]
- Stein PD, Henry JW. Prevalence of acute pulmonary embolism among patients in a general hospital and at autopsy. Chest 1995;108:978-81. [Crossref] [PubMed]
- Liew NC, Gul Y, Moissinac K. Postoperative Venous Thromboembolism in Asia: A Critical Appraisal of its Incidence. Asian J Surg 2003;26:154-8. [Crossref] [PubMed]
- Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e227S-77S.
- NCCN Guidelines. Venous Thromboembolic Disease. Version 2.2013, 04/19/13 © National Comprehensive Cancer Network.
- Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J 2008;29:2276-315. [Crossref] [PubMed]
- Gómez-Hernández MT, Rodríguez-Pérez M, Novoa-Valentín N, et al. Prevalence of Venous Thromboembolism in Elective Thoracic Surgery. Arch Bronconeumol 2013;49:297-302. [Crossref] [PubMed]
- Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e419S-96S.
- Ziomek S, Read RC, Tobler HG, et al. Thromboembolism in patients undergoing thoracotomy. Ann Thorac Surg 1993;56:223-6. [Crossref] [PubMed]
- Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the Emergency Department: The Revised Geneva Score. Ann Intern Med 2006;144:165-71. [Crossref] [PubMed]
- Donahue DM. Pulmonary Embolism Prophylaxis: Evidence for Utility in Thoracic Surgery. Thorac Surg Clin 2005;15:237-42. [Crossref] [PubMed]
- Egawa N, Hiromatsu S, Shintani Y, et al. Prevention of Venous Thromboembolism in Thoracic and Cardiovascular Surgery. Asian Cardiovasc Thorac Ann 2009;17:505-9. [Crossref] [PubMed]
- Mandalà M, Falanga A, Roila F, et al. Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 2011;22:vi85-92. [Crossref] [PubMed]
- Yoshida J, Inoue M, Furugaki K, et al. Pulmonary thromboembolism in lung surgery: use of unfractionated heparin. Asian Cardiovasc Thorac Ann 2014;22:46-8. [Crossref] [PubMed]
- Kakkar AK, Cimminiello C, Goldhaber SZ, et al. Low-Molecular-Weight Heparin and Mortality in Acutely Ill Medical Patients. N Engl J Med 2011;365:2463-72. [Crossref] [PubMed]
- Nagahiro I, Andou A, Aoe M, et al. Intermittent Pneumatic Compression Is Effective in Preventing Symptomatic Pulmonary Embolism after Thoracic Surgery. Surg Today 2004;34:6-10. [Crossref] [PubMed]
- Eppsteiner RW, Shin JJ, Johnson J, et al. Mechanical Compression versus Subcutaneous Heparin Therapy in Postoperative and Post-trauma Patients: A Systematic Review and Meta-Analysis. World J Surg 2010;34:10-9. [Crossref] [PubMed]


