Inflammation is related to preoperative hypoxemia in patients with acute Stanford type A aortic dissection
Introduction
Acute Stanford type A aortic dissection (ATAAD) is an age-dependent cardiovascular disease associated with high morbidity and mortality because of its potentially fatal complications (1,2). The overall mortality rate of ATAAD is about 10.3% in China (3). ATAAD not only involves the cardiovascular system but can cause multi-organ failure (4). Although the methods of diagnosis, detection, and treatment have been continuously improving, ATAAD remains one of the most severe diseases of the aorta (5). Patients with ATAAD often suffer from acute lung injury (ALI), and hypoxemia is the most typical symptom in patients with ALR. Hypoxemia is defined by an arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) ratio of 200 or lower (6). ALI is caused by various factors that result in injury to the alveolar epithelial and capillary endothelial cells, resulting in acute hypoxic respiratory insufficiency. The pathophysiological characteristics include the imbalance between ventilation and blood flow (6).
The mechanisms underlying preoperative hypoxemia and the cause of lung injury in ATAAD remain elusive. There have been no studies describing relationships between preoperative hypoxemia and ATAAD. In the present study, we established a model to predict the risk factors of ALR before surgery, and then tried to determine the predictors of preoperative hypoxemia, to improve early treatment of hypoxemia in ATAAD.
Materials and methods
Materials
Risk factors for preoperative hypoxemia
Included in this study were 172 consecutive patients with ATAAD who received treatment in the Department of Cardiothoracic Surgery at Shanghai Changhai Hospital between January 2013 and February 2015. The mean age of patients was 51.4±12.7 years, including 135 male patients. Preoperative hypoxemia was defined by an arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) ratio of 200 or lower. PaO2 was obtained from the arterial blood gas. FiO2 was equal to 21 plus 4× the oxygen flow (7).
The patients were retrospectively divided into group A (preoperative hypoxemia, n=77) and group B (preoperative non-hypoxemia, n=95).
Prospective study of hypoxemia before surgery
Included in this part of the study were 55 consecutive patients with ATAAD who received treatment in the Department of Cardiothoracic Surgery at Shanghai Changhai Hospital from February 2015 to September 2015. According to a computer-generated sequence, patients were prospectively randomized into two groups: the ulinastatin group (C group, n=17) and the Control group (D group, n=38). The ulinastatin group received ulinastatin at total doses of 300,000 units before surgery. All the patients underwent cardiopulmonary bypass with induction of deep hypothermic circulatory arrest.
Methods
We collected patient data including age, sex, height, weight, current smoking, current drinking, and history of hypertension, diabetes, chronic obstructive pulmonary disease (COPD), coronary disease, and Marfan syndrome. White blood cell (WBC) counts, neutrophil and platelet counts, and alanine aminotransferase (ALT), glutamic oxaloacetic transaminase (AST), C-reactive protein (CRP), D-dimer, prothrombin time (PT), creatinine (Cr) and interleukin-6 (IL-6) levels were all measured in our laboratory. The left ventricular ejection fraction (LVEF), presence of pericardial effusion, presence of pleural effusion and presence of aortic valve insufficiency were determined via echocardiography. Contrast-enhanced aortic computed tomography (CT) was used to see whether dissection involved the celiac trunk, renal artery (unilateral or bilateral), or mesenteric artery. These data were collected before surgery.
Patient data were collected during surgery including the ECCT (extracorporeal circulation time), AACT (arrest aortic clamping time), and the DHCA (deep hypothermic circulatory arrest). Red cell concentrations for the patients were calculated during surgery and in the first 24 hours postoperatively.
This study was approved by Changhai Hospital Clinical Research Ethics Committee (2016LC0118-08).
Statistical analysis
All statistical analyses were performed using the SPSS 21.0 software package. The continuous data are expressed as mean ± SD. The normally distributed variables were analyzed using the Student’s t-test, and the non-normally distributed variables were analyzed using the Wilcoxon rank sum test. The qualitative data were analyzed using the chi-square test or Fisher’s exact test. P<0.05 was considered statistically significant. If single factors between the two groups were statistically significant, then these factors were tested using the multivariate binary logistic regression analysis to predict the independent risk factors. The odds ratios (OR) and the 95% confidence intervals (CIs) were also calculated.
Results
Risk factors for preoperative hypoxemia (retrospective analysis)
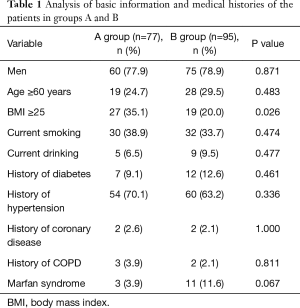
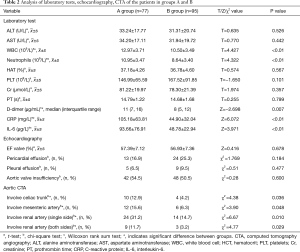
According to the level of preoperative hypoxemia, the 172 consecutive patients were retrospectively divided into group A (preoperative hypoxemia, n=77) and group B (preoperative non-hypoxemia, n=95). As shown in Tables 1 and 2, the data were collected, including age, sex, height, weight, current smoking, current drinking, and history of hypertension, diabetes, coronary disease, and Marfan syndrome. In addition, WBC, neutrophil and PLT counts, and ALT, AST, CRP, D-dimer, PT, Cr and IL-6 levels were determined in our laboratory. LVEF, presence of pericardial effusion, presence of pleural effusion, and presence of aortic valve insufficiency were determined by echocardiography. Contrast-enhanced CT of the aorta was used to determine if the dissection involved the celiac trunk, the renal artery, or the mesenteric artery. It was found that BMI ≥25, WBC and neutrophil counts, CRP, D-dimer and IL-6 levels, and dissection involving the celiac trunk, renal artery, or mesenteric artery were all associated with preoperative hypoxemia in ATAAD. Multivariate binary logistic regression analysis confirmed that serum CRP levels (OR 1.034, CI, 1.008–1.061; P=0.010) and IL-6 (OR 1.050; CI, 1.003–1.100; P=0.036) levels were independently associated with preoperative hypoxemia in ATAAD (Table 3).
Full table
Full table

Full table
Protective effect of ulinastatin on patients with ATAAD (prospective study)
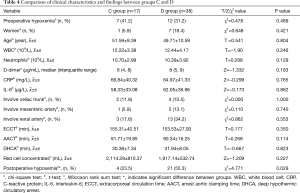
A prospective study was conducted next, in which 55 patients were randomized into two groups: the ulinastatin group (C group, n=17) and the control group (D group, n=38). As shown in Table 4, the relative facts during the perioperative period were no different between the two groups. However, postoperative hypoxemia was significantly different (χ2=4.771; P=0.029).
Full table
Discussion
ATAAD can cause fatal complications and high mortality (8). Surgery remains the primary treatment option (8). Dissection complicated by lung injury, especially after surgery, is not only life-threatening, but may prolong the length of ventilator support and the stay in the ICU (9).
In our study, according to the diagnostic criteria for acute respiratory distress syndrome (ARDS) established by the American–European Consensus Conference, hypoxemia was defined as a PaO2/FiO2 ratio ≤200 (P/F ratio) (10). Patients with ATAAD often have hypoxemia before surgery. The results of our study showed that preoperative hypoxemia was associated with a variety of factors.
Obesity by itself could easily induce respiratory system-related diseases (11) and is a known of cause obstructive sleep apnea and obesity hypoventilation syndrome. These diseases and disorders are closely associated with hypoxemia. Aizawa et al. (12) found that obesity was a risk factor for young-onset ATAAD and postoperative hypoxemia, both of which could prolong the length of intubation and ICU stay. The high-fat content in the pleura and chest walls in obese patients can reduce chest wall and lung compliance, lung volume and thoracic breathing and limit diaphragmatic activity, eventually leading to hypoxia (13). Therefore, obesity can be a potential risk factor used to predict hypoxemia (14). BMI ≥25 kg/m2 could easily lead to hypoxemia. To prevent pulmonary atelectasis in obese patients, sputum aspiration should be performed positively during and after surgery. Maintaining airway patency and performance of regular X-ray examinations are also needed.
When ATAAD occurs, the coagulation and fibrinolytic systems are activated. Fibrinogen turns into fibrin which participates in the formation of a thrombus. Increased circulating D-dimer levels are reported to be correlated with adverse outcomes in various clinical settings (15). The detection interval of the D-dimer test in our hospital is 0.01–16 µg/mL, and therefore only D-dimer levels above 16 µg/mL were counted in this study. We know that the normal level of serum D-dimer is less than 0.5 mg/L. The D-dimer level in all 172 patients was positive (sensitivity of 100%). Sbarouni et al. (16) found that a serum D-dimer level higher than 700 ng/mL had 94% sensitivity and 59% specificity for the diagnosis of ATAAD. D-dimer ≥5.67 µg/mL was an important risk factor and was independently associated with ATAAD inpatient deaths (17). D-dimer is a marker of fibrinolysis and coagulation (18). D-dimer may reflect disease severity in ATAAD patients with preoperative hypoxemia.
ATAAD involving the celiac trunk, renal artery (unilateral or bilateral) or mesenteric artery is a risk factor for patients with ATAAD and preoperative hypoxemia. When an ATAAD is formed, large amounts of blood flow into the false lumen. With the expansion of the dissection, more blood flows into it. This can lead to ventilation/perfusion ratio imbalances. The percentage of ATAAD is defined as the percentage of blood volume in the false lumen to that of the blood in the aorta (19) There is a negative correlation between the ventilation/perfusion ratio and AAD% (r=−0.604) (19). The AAD% in patients with preoperative hypoxemia was higher than that in patients without preoperative hypoxemia (50.8%±10.9% vs. 28.0%±11.9%, P<0.01). Systemic inflammatory reactions are associated with this process (19).
Systemic inflammatory reactions play a vital role in this process after the onset of ATAAD. Inflammatory reactions are involved in the development of ATAAD (20). Systemic inflammation can be caused by the ATAAD. Increased plasma inflammatory markers are believed to be significantly associated with ATAAD (21). In our study, we found that the WBC and neutrophil counts were increased, and the serum CRP and IL-6 levels were elevated significantly in patients with ATAAD. A multivariate binary logistic regression analysis confirmed that they were independently associated with preoperative hypoxemia in ATAAD.As an acute phase reactant, CRP is a sensitive and non-specific inflammatory marker (22). CRP ≥11.21 mg/L was a significant risk factor for preoperative hypoxemia and was independently associated with an inpatient risk of death in ATAAD patients (17). Prolonged elevation or re-elevation of CRP may be an important factor to guide clinical interventions (23). IL-6 is known to be secreted at high levels in human aortic disease (24). Tieu et al. reported that IL-6production contributes to vascular inflammation which can lead to aneurysm and dissection (25). Plasma concentrations of IL-6 were tested in 64 patients with ATAAD, 98 patients with hypertension alone, and 96 healthy subjects (10.98±2.38 vs. 3.79±1.56 and 3.32±1.60 pg/mL, P<0.05, respectively) (20). The expression of IL-6 was obvious in ALR. The IL-6 level in the serum and bronchoalveolar lavage fluid rose significantly in patients with pulmonary injury (26). ATAAD can cause systemic inflammatory response. With the self-destruction and cascade amplification, the inflammatory reaction increases gradually. The inflammatory substances are activated in the lung, which can destroy the integrity of the alveolar cells, eventually leading to pulmonary edema. The extensive existence of capillary beds in the lung tissue is likely to cause injury.
The early use of beta-blockers could prevent excessive inflammation after ATAAD (27). Appropriate clinical interventions may improve oxygenation, so a further prospective study was conducted. Fifty-five patients were prospectively randomized into two groups: the ulinastatin group (C group, n=17) and the control group (D group, n=38). Compared to the control group, the postoperative hypoxemia of patients in the ulinastatin group was significantly improved. Ulinastatin is a broad-spectrum protease inhibitor. It has been used widely in patients with acute inflammatory disorders (28). Ulinastatin stabilizes the lysosomal membrane and inhibits the release of hydrolytic enzymes in vivo. It also inhibits the generation of myocardial inhibitory factor, reducing the production of oxygen free radicals and inhibiting the excessive release of various inflammatory mediators. It is widely used in the treatment of SIRS and other diseases (29,30). The protective mechanism of ulinastatin can be attributed to the inhibited production of pro-inflammatory cytokines (31), The effect of ulinastatin on respiratory function after CPB under DHCA could improve oxygenation (32).
However, the mechanism of preoperative hypoxemia remains unclear. To the best of our knowledge, there is no published study reporting on patients with ATAAD and preoperative hypoxemia. Generally, long- term smoking and smoking history can damage alveolar epithelial cells and the trachea and bronchial epithelial cells, resulting in pulmonary function damage (33). However, we failed to find a significant difference between the two groups in our study. Our research shows that acute inflammatory response may play a vital role in this process.
Conclusions
Hypoxemia is a complication in patients with ATAAD. BMI, systemic inflammatory response, D-dimer level, and the extent of dissection are risk factors for preoperative hypoxemia, of which inflammatory response is the independent predictive factor. Appropriate clinical interventions may play a virtual role in the prevention and treatment of hypoxemia in the perioperative period.
Acknowledgements
Funding: This work was sponsored by Wu Jieping Medical Foundation (320.6750.16093) and Natural Science Foundation of Shanghai (16ZR1400900).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by Changhai Hospital Clinical Research Ethics Committee (2016LC0118-08).
References
- De León Ayala IA, Chen YF. Acute aortic dissection: an update. Kaohsiung J Med Sci 2012;28:299-305. [Crossref] [PubMed]
- Algarni KD, Yanagawa B, Rao V, et al. Profound hypothermia compared with moderate hypothermia in repair of acute type A aortic dissection. J Thorac Cardiovasc Surg 2014;148:2888-94. [Crossref] [PubMed]
- Wang W, Duan W, Xue Y, et al. Clinical features of acute aortic dissection from the Registry of Aortic Dissection in China. J Thorac Cardiovasc Surg 2014;148:2995-3000. [Crossref] [PubMed]
- Hughes GC, Ganapathi AM, Keenan JE, et al. Thoracic endovascular aortic repair for chronic DeBakey IIIb aortic dissection. Ann Thorac Surg 2014;98:2092-7; discussion 2098. [Crossref] [PubMed]
- Wilbring M, Ghazy T, Matschke K, et al. Complete endovascular treatment of acute proximal ascending aortic dissection and combined aortic valve pathology. J Thorac Cardiovasc Surg 2015;149:e59-60. [Crossref] [PubMed]
- Johnson ER, Matthay MA. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv 2010;23:243-52. [Crossref] [PubMed]
- Hui D, Morgado M, Chisholm G, et al. High-flow oxygen and bilevel positive airway pressure for persistent dyspnea in patients with advanced cancer: a phase II randomized trial. J Pain Symptom Manage 2013;46:463-73. [Crossref] [PubMed]
- Di Bartolomeo R, Pantaleo A, Berretta P, et al. Frozen elephant trunk surgery in acute aortic dissection. J Thorac Cardiovasc Surg 2015;149:S105-9. [Crossref] [PubMed]
- Hysi I, Juthier F, Fabre O, et al. Aortic root surgery improves long-term survival after acute type A aortic dissection. Int J Cardiol 2015;184:285-90. [Crossref] [PubMed]
- Ho VP, Madbak F, Horng H, et al. Analysis of Hypoxemia in Early Ventilator-Associated Pneumonia Secondary to Haemophilus in Trauma Patients. Surg Infect (Larchmt) 2015;16:293-7. [Crossref] [PubMed]
- Pereira H, Xara D, Mendonca J, et al. Patients with a high risk for obstructive sleep apnea syndrome: postoperative respiratory complications. Rev Port Pneumol 2013;19:144-51. [Crossref] [PubMed]
- Aizawa K, Sakano Y, Ohki S, et al. Obesity is a risk factor of young onset of acute aortic dissection and postoperative hypoxemia. Kyobu Geka 2013;66:437-44. [PubMed]
- Mahadev S, Salome CM, Berend N, et al. The effect of low lung volume on airway function in obesity. Respir Physiol Neurobiol 2013;188:192-9. [Crossref] [PubMed]
- Lumachi F, Marzano B, Fanti G, et al. Relationship between body mass index, age and hypoxemia in patients with extremely severe obesity undergoing bariatric surgery. In Vivo 2010;24:775-7. [PubMed]
- Turak O, Canpolat U, Ozcan F, et al. D-dimer level predicts in-hospital mortality in patients with infective endocarditis: a prospective single-centre study. Thromb Res 2014;134:587-92. [Crossref] [PubMed]
- Sbarouni E, Georgiadou P, Marathias A, et al. D-dimer and BNP levels in acute aortic dissection. Int J Cardiol 2007;122:170-2. [Crossref] [PubMed]
- Wen D, Du X, Dong JZ, et al. Value of D-dimer and C reactive protein in predicting in hospital death in acute aortic dissection. Heart 2013;99:1192-7. [Crossref] [PubMed]
- Lin CH, Chen SC, Chen WJ, et al. Dimerized plasmin fragment D: a reliable biomarker for diagnosing aortic dissection? Am J Emerg Med 2010;28:121.e1-3. [Crossref] [PubMed]
- Kurabayashi M, Okishige K, Azegami K, et al. Reduction of the PaO2/FiO2 Ratio in Acute Aortic Dissection. Circ J 2010;74:2066-73. [Crossref] [PubMed]
- Luo F, Zhou XL, Li JJ, et al. Inflammatory response is associated with aortic dissection. Ageing Res Rev 2009;8:31-5. [Crossref] [PubMed]
- Wen D, Zhou XL, Li JJ, et al. Plasma concentrations of interleukin-6, C-reactive protein, tumor necrosis factor-alpha and matrix metalloproteinase-9 in aortic dissection. Clin Chim Acta 2012;413:198-202. [Crossref] [PubMed]
- Wen D, Zhou XL, Li JJ, et al. Biomarkers in aortic dissection. Clin Chim Acta 2011;412:688-95. [Crossref] [PubMed]
- Okina N, Ohuchida M, Takeuchi T, et al. Utility of measuring C-reactive protein for prediction of in-hospital events in patients with acute aortic dissection. Heart Vessels 2013;28:330-5. [Crossref] [PubMed]
- Treska V, Topolcan O, Pecen L. Cytokines as plasma markers of abdominal aortic aneurysm. Clin Chem Lab Med 2000;38:1161-4. [Crossref] [PubMed]
- Tieu BC, Lee C, Sun H, et al. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest 2009;119:3637-51. [Crossref] [PubMed]
- Bhargava M, Wendt CH. Biomarkers in acute lung injury. Transl Res 2012;159:205-17. [Crossref] [PubMed]
- Jo Y, Anzai T, Sugano Y, et al. Early use of beta-blockers attenuates systemic inflammatory response and lung oxygenation impairment after distal type acute aortic dissection. Heart Vessels 2008;23:334-40. [Crossref] [PubMed]
- Zhao G, Zhu Y, Yu D, et al. The effect of ulinastatin on hyperglycemia in patients undergoing hepatectomy. J Surg Res 2015;193:223-8. [Crossref] [PubMed]
- Wang WK, Lu QH, Wang X, et al. Ulinastatin attenuates diabetes induced cardiac dysfunction by the inhibition of inflammation and apoptosis. Exp Ther Med 2017;14:2497-504. [Crossref] [PubMed]
- Pan Y, Fang H, Lu F, et al. Ulinastatin ameliorates tissue damage of severe acute pancreatitis through modulating regulatory T cells. J Inflamm (Lond) 2017;14:7. [Crossref] [PubMed]
- Li W, Qiu X, Jiang H, et al. Ulinastatin inhibits the inflammation of LPS-induced acute lung injury in mice via regulation of AMPK/NF-kappaB pathway. Int Immunopharmacol 2015;29:560-7. [Crossref] [PubMed]
- Xu CE, Zou CW, Zhang MY, et al. Effects of high-dose ulinastatin on inflammatory response and pulmonary function in patients with type-A aortic dissection after cardiopulmonary bypass under deep hypothermic circulatory arrest. J Cardiothorac Vasc Anesth 2013;27:479-84. [Crossref] [PubMed]
- Arabaci U, Akdur H, Yigit Z. Effects of smoking on pulmonary functions and arterial blood gases following coronary artery surgery in Turkish patients. Jpn Heart J 2003;44:61-72. [Crossref] [PubMed]


