Cannulation for veno-venous extracorporeal membrane oxygenation
Introduction
Extracorporeal membrane oxygenation (ECMO) is described as a modified, smaller cardiopulmonary bypass circuit that is used for a short period (days to weeks) in patients with acute cardiac and/or respiratory failure refractory to conventional management (1). The Extracorporeal Life Support Organization (ELSO) present different modes of ECMO, veno-venous (VV) and veno-arterial (VA) being the two basic principles (2). The VV ECMO circuit drains venous blood, oxygenate the blood, and pump the blood back into the same venous compartment. That means that the VV ECMO circuit is totally separated from the patients’ cardiac output (CO). With no CO the pump flow in the ECMO circuit will result in 100% recirculation. Cardiac function and CO is needed to deliver oxygen to the tissues. The VA ECMO circuit drain venous blood, oxygenate and pump the blood back into the arterial vascular compartment, therefore not dependent on cardiac function and CO. VA ECMO can thus replace CO completely and deliver oxygen to tissues without cardiac function. VV ECMO is an established method and when used in experienced ECMO centers it improves survival in patients suffering from severe ARDS or respiratory insufficiency (3-7).
The goal is to drain the least saturated blood. If draining low saturated blood, it is possible to add oxygen up to 100% saturation, before pumping the blood back to the patient. Delivery of oxygen to the patient is dependent of saturation of the blood drained from the patient, hemoglobin concentration and pump flow. Therefore, draining the least saturated blood will produce the most efficient ECMO circuit. The saturation in superior vena cava (SVC) and inferior vena cava (IVC) are almost the same in the intensive care patient. The right atrium, RA, and pulmonary artery, PA, have lower saturation than SVC and IVC, because of mixing with venous blood from coronary sinus (8). In ECMO literature and on ECMO pumps the venous blood drained to the ECMO pump is called “SvO2”, mixed venous saturation. But it has nothing to do with the real mixed venous saturation in the pulmonary artery, SvO2. A better nomenclature for the venous blood drained to the pump is pre-oxygenator saturation.
Overview
Why the draining venous cannula is the most important part of the ECMO circuit.
A tube is creating a flow resistance known thru Poiseuille equation published in 1840, ∆P = (8 Viscosity Length Flow)/(π Radius4). The formula applied to the draining cannula indicates that the shortest possible cannula with the biggest possible diameter will create the least resistance and provide the best drainage. The draining cannula will define the maximum flow in the circuit due to resistance and will also define the efficiency of the system depending on the pre-oxygenator saturation.
Recirculation during VV ECMO
Definition: reinfused oxygenated blood is withdrawn by the drainage cannula without passing through the systemic circulation, decreasing the efficiency. Draining blood in the middle of RA will result in variable degrees of recirculation. The draining position within the venous system, ECMO pump flow, return flow position within the venous system and CO all have an impact on recirculation (9-11).
Dual cannula single lumen cannulation strategy
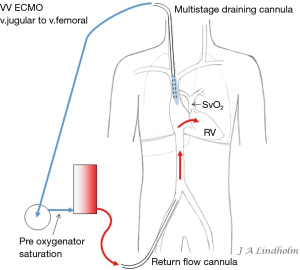
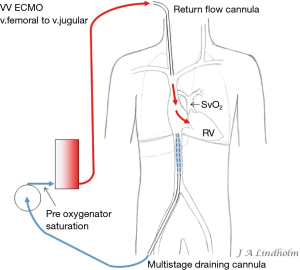
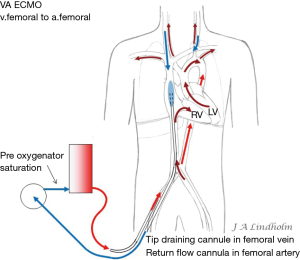
Given the above with the same saturation in SVC as in IVC it gives the option of draining from IVC and return saturated blood in SVC or draining from SVC and return saturated blood in IVC. To avoid or minimize recirculation the draining point should be far away from the inflow into the right ventricle (RV). Draining in the IVC inferior of the diaphragm letting saturated blood flow from SVC into RV or draining in the SVC letting the saturated blood from IVC flow into the heart and not into draining cannula (10,11) (Figures 1,2).

Dual lumen single cannula cannulation
A dual lumen cannula is inserted via the right jugular vein and drain from SVC and lower RA and reinfuse in the middle of RA towards the tricuspid valve, this cannula design is common in neonatal ECMO and will cause a variable degree of recirculation depending on ECMO pump flow. A different version of dual lumen cannula draining both from SVC and from IVC and reinfuse in RA towards tricuspid valve can result in very low recirculation if in an anatomically good position. If a dual lumen cannula is malpositioned the reinfused flow can cause severe turbulence and very high degree of recirculation. The tip of a dual lumen cannula can also cause damage to atrium or ventricle if malposition and needs monitoring by ECHO (11).
Cannula designs
Tip draining design
The design of the venous cannula will have big impact on where the cannula will drain blood within the venous system. A cannula draining only in the tip will drain blood around the tip (Figure 1).
Multistage draining design
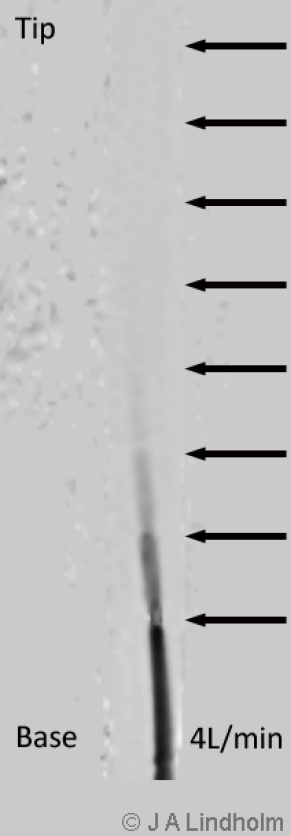
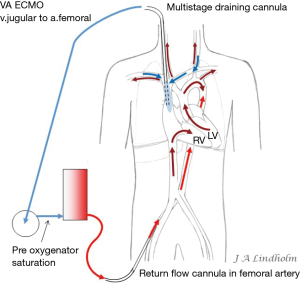
There are multiple stages draining cannulas available for cannulation via the femoral vein with side holes draining as far as 20 cm from the tip (Figure 3). Depending on if these side holes are blocked or not by a collapsed inferior caval vein, IVC, the draining point within the venous system can differ 20 cm due to Poiseuille equation on flow resistance. The problem is explained by a MRI velocity scan in a non-wired copy of a multistage cannula (Figure 4). At pump flow of 4 L/min and with all the side holes open the cannula is mainly draining from the most proximal holes and almost nothing at the tip. The draining point within IVC can therefore be 15 cm below diaphragm even if the tip is in RA. There are shorter multiple stage cannulas available draining from side holes draining as far as 9 cm from the tip (Figure 2).
Expanding basket design
There are also draining cannulas designed for insertion in the femoral vein with a net basket that expands inside the lower part of IVC to prevent the IVC from collapsing around the cannula.
VV ECMO is a dynamic treatment
Reduced or increased compliance of the lungs will alter the position of the diaphragm. Consolidated lungs, pleura effusion, pneumothorax, hemothorax and breathing effort can also change the anatomy and position of a cannula. Due to the position of the diaphragm a cannula in perfect position one day might be malpositioned the next day, even though the cannula is secured in the same position at the incision level. High or low pressures in the ventilator will prevent or permit blood flow into the thoracic cavity, leading to variations of blood volume in the IVC which can have an impact on venous drainage if the draining point is in the IVC (11).
Pulmonary vascular resistance (PVR)
Pulmonary vasoconstriction is a normal physiological response to hypoxia in the alveoli responsible for reducing pulmonary blood flow to non-ventilated alveoli and therefore avoiding desaturation (12-16).
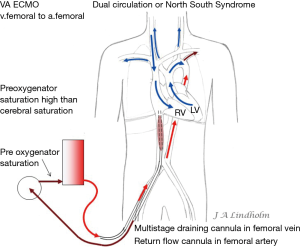
In the case of bronchopneumonia involving a lobe or a lung this vasoconstriction redirects the blood to ventilated lung tissue. General hypoxemia has no effect on pulmonary vasodilatation and PVR will remain high if there is no oxygen in the alveoli (17). When using VV ECMO often both lungs are non-ventilated due to infection and inflammation, which will cause increasing PVR in a variable degree. VV ECMO therefore presents a risk of right cardiac failure due to high PVR (18). In this situation with a failing CO due to right heart failure there is a possibility to convert from VV ECMO to VA ECMO (19). Depending on where the draining point is within the venous system this can result in a dual circulation situation with severe desaturation of the upper body including the brain, known as dual circulation or “North South Syndrome” (20,21).
Dual circulation when converting from VV to VA ECMO
Dual circulation or “North South Syndrome” occurs on VA ECMO when draining from the IVC and reinfusing oxygenated blood into the femoral artery while there is a pulmonary blood flow without any oxygenation by the lungs. Blood from SVC enter RV and pulmonary artery, and then non-oxygenated blood from the pulmonary veins enters the left ventricle and is ejected into proximal aorta and cerebral arteries. Reinfused oxygenated blood from the ECMO pump enter distal aorta and support abdominal organs and legs. The oxygen consumption in the lower part of body is low and saturation in the IVC will therefore be high in this situation resulting in high pre-oxygenator saturation, the “mixed venous saturation” on the ECMO pump “SvO2” can be as high as 80–90%, while at the same time the saturation in the right hand or the patient’s ears can measure as low as 40% (Figure 5). High pre-oxygenator saturation also means low efficiency. Dual circulation can lead to a lethal situation with very low cerebral PO2 (21). Adding a third cannula in the jugular vein to reinfuse oxygenated blood can solve the problem with cerebral hypoxia but draining the desaturated blood in SVC is always better for the efficiency of the circuit and also solve the dual circulation problem (21-23). Dividing flow using a Y connector also increases the risk of clotting and should be avoided.
Note that in both VV and VA ECMO the blood that enters the RV will be the blood delivered to the brain if there is a pulmonary blood flow. With no gas exchange within the lungs the cerebral arterial saturation will be the same as SvO2. When the ECMO pump flow does not reach the brain during VA ECMO, the goal is to raise the real SvO2 as high as possible by draining the lower saturated blood in SVC and letting the higher saturated blood in IVC enter the RV (Figures 6,7).
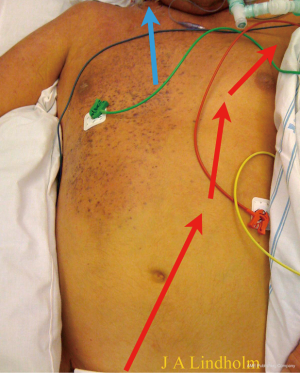
The cerebral saturation will be the same as real SvO2 and the lower body saturation will be 100%, this is not dual circulation but normal physiology during VA ECMO and is displayed in a patient with cerebral saturation of 80%, left hand saturation 95%, lower body saturation 100% and pre-oxygenator saturation 70–75% (Figure 8). The chest wall arterial support origins from subclavian artery, therefore the right side of the chest has saturation of 80% and the left side have higher saturation since pump flow meets native flow and mix in that area in this patient. A situation with both very low cerebral saturation and very low pre-oxygenator saturation means there is not enough pump flow or total oxygen delivery.
If draining venous blood from the SVC, the dual circulation will never occur when converting from VV to VA ECMO. The dual circulation can also be avoided by open chest central cannulation with a draining cannula in RA and with the return flow cannula in the ascending aorta, but good drainage and avoiding dual circulation can be achieved using correct peripheral cannulas.
Applying the rule of draining the least saturated venous blood, conclude that the use of multistage cannulas inserted into the femoral vein or cannulas draining only from IVC is not suitable for conversion from VV to VA ECMO (Figure 5). Draining cannulas inserted into the femoral vein should be tip draining so they can be advanced up to upper part of RA in a situation with dual circulation (Figure 6). Using a short multistage cannula inserted into the right jugular vein as draining cannula will need no adjustment when converting from VV to VA ECMO (Figure 2). A short cannula inserted into the jugular vein provides excellent flow due to low flow resistance.
Conclusions
VV ECMO can be performed with different cannulation strategies. Using two single lumen cannulas draining in the IVC and reinfusing in the SVC, draining in the SVC and reinfusing in the IVC, or dual lumen cannulas inserted in right jugular vein are all working strategies. Independent of cannulation strategy there will be a risk of recirculation during VV ECMO. But efficiency can be good or reasonable in either strategy if cannulas are carefully positioned and monitored during the dynamic procedure of pulmonary disease.
The disadvantage draining from IVC only occurs when converting from VV to VA ECMO and reinfusing in the femoral artery. Then draining from SVC is the most efficient strategy, draining low saturated venous blood, and also means minimal risk of dual circulation. Converting from VV to VA ECMO while draining from the SVC is simple, it only needs addition of an arterial femoral cannula.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Cardiovascular Pathology (Fourth Edition), 2016.
- Extracorporeal Life Support Organization, ELSO. Available online: https://www.elso.org/resources/guidelines.aspx
- Peek GJ, Moore HM, Moore N, et al. Extracorporeal Membrane Oxygenation for Adult Respiratory Failure. Chest 1997;112:759-64. [Crossref] [PubMed]
- Peek GJ, Sosnowski AW. Extra-corporeal membrane oxygenation for paediatric respiratory failure. Br Med Bull 1997;53:745-56. [Crossref] [PubMed]
- Pettignano R, Fortenberry JD, Heard ML, et al. Primary use of the veno venous approach for extracorporeal membrane oxygenation in pediatric acute respiratory failure. Pediatr Crit Care Med 2003;4:291-8. [Crossref] [PubMed]
- Knight GR, Dudell GG, Evans ML, et al. A comparison of veno venous and veno arterial extracorporeal membrane oxygenation in the treatment of neonatal respiratory failure. Crit Care Med 1996;24:1678-83. [Crossref] [PubMed]
- Hayes D Jr, Tobias JD, Kukreja J, et al. Extracorporeal life support for acute respiratory distress syndromes. Ann Thorac Med 2013;8:133-41. [Crossref] [PubMed]
- Gutierrez G, Venbrux A, Ignacio E, et al. The concentration of oxygen, lactate and glucose in the central veins, right heart, and pulmonary artery: a study in patients with pulmonary hypertension. Crit Care 2007;11:R44. [Crossref] [PubMed]
- Xie A, Yan TD, Forrest P. Recirculation in veno venous extracorporeal membrane oxygenation. J Crit Care 2016;36:107-10. [Crossref] [PubMed]
- Palm10 O, PalmPa K, Hultman J, et al. Cannula Design and Recirculation During Veno venous Extracorporeal Membrane Oxygenation. ASAIO J 2016;62:737-42.
- Abrams D, Bacchetta M, Brodie D. Recirculation in venovenous extracorporeal membrane oxygenation. ASAIO J 2015;61:115-21. [Crossref] [PubMed]
- Dirken MN, Heemstra H. Alveolar oxygen tension and lung circulation. Q J Exp Physiol 1948;34:193-211. [Crossref] [PubMed]
- Doyle JT, Wilson SJ, Warren JV. The Pulmonary Vascular Responses to Short-Term Hypoxia in Human Subjects. Circulation 1952;5:263-70. [Crossref] [PubMed]
- Duke HN, Killick EM. Pulmonary vasoconstriction to anoxia: its site of action. J Physiol 1952;117:78P-79P. [PubMed]
- Duke HN. The site of action of anoxia on the pulmonary blood vessels of the cat. J Physiol 1954;125:373-82. [Crossref] [PubMed]
- Sylvester JT, Mitzner W, Ngeow Y, et al. Hypoxic constriction of alveolar and extra-alveolar vessels in isolated pig lungs. J Appl Physiol Respir Environ Exerc Physiol 1983;54:1660-6. [PubMed]
- Rounds SI, Moore LG, Voelkel NF, et al. Cardiac output is decreased and hypoxic vasoconstriction is intact in chronically hypoxic sheep. Proc Soc Exp Biol Med 1980;165:1-5. [Crossref] [PubMed]
- Ius F, Sommer W, Tudorache I, et al. Veno-veno-arterial extracorporeal membrane oxygenation for respiratory failure with severe haemodynamic impairment: technique and early outcomes. Interact Cardiovasc Thorac Surg 2015;20:761-7. [Crossref] [PubMed]
- Dong ER, Ng DG, Ramzy D, et al. Acute Cor Pulmonale in Veno-Venous Extracorporeal Membrane Oxygenation: Three Case Reports. ASAIO J 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Lindfors M, Frenckner B, Sartipy U, et al. Venous Cannula Positioning in Arterial Deoxygenation During Veno-Arterial Extracorporeal Membrane Oxygenation-A Simulation Study and Case Report. Artif Organs 2017;41:75-81. [Crossref] [PubMed]
- Hou X, Yang X, Du Z, et al. Superior vena cava drainage improves upper body oxygenation during veno-arterial extracorporeal membrane oxygenation in sheep. Crit Care 2015;19:68. [Crossref] [PubMed]
- Geyer M, Gohrbandt B, Sagoschen I, et al. Pitfalls of cannulation for extracorporeal life support: review of the literature and illustrative case presentation. J Artif Organs 2018;21:8-16. [Crossref] [PubMed]
- Napp LC, Kühn C, Hoeper MM, et al. Cannulation strategies for percutaneous extracorporeal membrane oxygenation in adults. Clin Res Cardiol 2016;105:283-96. [Crossref] [PubMed]


