Appropriate lymphadenectomy significantly reduced recurrence after segmentectomy for patients with non-small cell lung cancer
Introduction
The diagnosis of early-stage non-small cell lung cancer (NSCLC) has dramatically increased during the past ears, thanks to the widely use of low-dose computed tomography (CT). Patients with clinical stage IA lung cancer constituted as high as 36% of all patients in the database maintained by the International Association for the Study of Lung Cancer (IASLC) to inform the eighth edition TNM classification (1). Lobectomy combined with systematic lymph node (LN) dissection or sampling currently is the treatment of choice for patients with NSCLC. However, increasing evidence has emerged from institutional case series and national registry to support the equivalency in the long-term survival between segmentectomy and lobectomy for small-sized early-stage NSCLC (2-6). Segmentectomy is chosen for the well-selected patients, for its advantage of preserving lung parenchyma, pulmonary function and a chance of future resections for secondary primary lung cancer (7). An investigation on the intrapulmonary tumor spread pattern showed that metastasis outside the tumor-bearing segment was rarely observed in small-sized NSCLC (8). Segmentectomy can remove the intrapulmonary lymphatic drainage along with the pulmonary segment, leading to the advantage of more extensive lymphadenectomy over wedge resection, another surgical type of sublobar resection.
Lymphatic metastasis is the major route of dissemination for NSCLC and can occur in the early phage of NSCLC (9). For patients undergoing lobectomy for NSCLC, accumulating data have demonstrated that a greater number of harvested LNs is associated with improved survival (10,11). Normally, only patients with node-negative disease in preoperative clinical staging are deemed to be suitable candidates for segmentectomy. It remains unknown whether lymphadenectomy is necessary for early-stage NSCLC patients undergoing segmentectomy. The current National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines recommends that the appropriate LN stations should be sampled for patients undergoing sublobar resection, unless not technically feasible (12). Nevertheless, there is still a lack of direct evidence demonstrating the clinical significance of lymphadenectomy for patients undergoing segmentectomy.
Our center, Shanghai Chest Hospital, in which a total of 10,562 major thoracic operations were performed in the calendar year of 2016, is one of the largest thoracic surgery centers worldwide (13). The ultra-high volume provides a great opportunity to maintain a large cohort of patients undergoing segmentectomy for clinical research. Herein, we retrospectively collected the clinicopathologic and surgical data of 259 patients undergoing segmentectomy and performed survival analyses, to investigate whether appropriate lymphadenectomy could provide survival benefits in the context of segmentectomy.
Methods
Study population
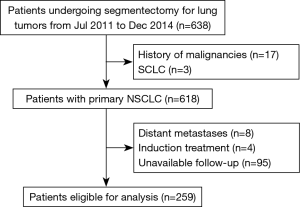
From July 2011 to December 2014, 638 patients undergoing segmentectomy for lung tumors at Shanghai Chest Hospital were retrospectively analyzed. Patients’ demographic and clinical data, disease extent, treatment and follow-up information were collected. Patients diagnosed with history of malignancies (including metastatic tumors in lung), small cell lung cancer and distant metastases were ineligible for the analysis. We also excluded those who received induction treatments prior to resection, and who were lost to follow-up after operation. Atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA) are considered to be free from LN metastasis (14), so patients with these lesions were excluded in our study. The present study was approved by the institutional review board.
The preoperative work-up was conducted in accordance with the institutional protocols to confirm whether the patient was clinically fit for operation. Briefly, patients received bronchoscopic examination, pulmonary function test, chest and upper abdominal CT scanning, and brain magnetic resonance imaging (MRI, preferred) or CT scanning. Positron emission tomography (PET)-CT scanning and nuclear medicine bone scan were applied when clinically indicated.
The resected lung specimens and LNs were fixed in 10% formalin and then embedded in paraffin. Subsequently, the hematoxylin and eosin (HE)-stained sections were evaluated microscopically by experienced pulmonary pathologists. The comprehensive histological subtyping system was used to semi-quantitatively record the components of each histological pattern (lepidic, acinar, papillary, micropapillary, solid and mucinous) in 5% increment for adenocarcinomas, and the predominant pattern was defined as the most dominant pattern. When the status of visceral pleural invasion (VPI) couldn’t be determined in HE-stained slides, Elastica-Masson staining was applied. All patients were re-staged using the eighth edition of the American Joint Committee on Cancer/Union for International Cancer Control TNM classification for lung cancer (1).
Statistical analyses
The categorical and continuous variables between two groups were compared by χ2-test and independent sample t-test as appropriate. The primary endpoint was recurrence-free survival (RFS), to reduce bias introduced by factors, such as post-recurrence treatment. RFS as defined as the interval between the date of operation and the date of any disease recurrence, death or the last follow-up. Overall survival (OS) was defined as the interval between the date of operation and the date of death from any cause or the last follow-up. Survival curves were depicted by Kaplan-Meier method, and compared by log-rank test among groups. Multivariable Cox proportional hazards regression model was applied to identify the independent predictors for survival. The statistical analyses were conducted using the SPSS Statistics (version 22; IBM, NY, USA). All the tests were two-sided, and the level of significance was set at P<0.05.
Results
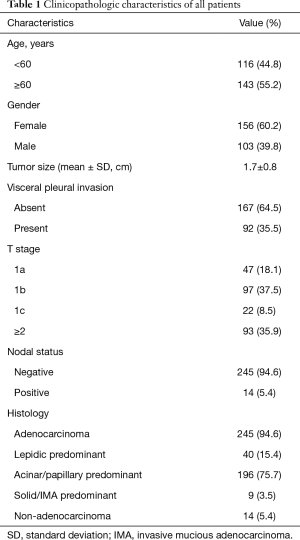
A total of 259 patients undergoing segmentectomy for NSCLC were eligible for our analysis. The patients’ selection process was shown in Figure 1. Table 1 displayed the demographic and clinicopathologic characteristics of these patients. There were more elder patients (55.2%) and more female patients (60.2%). The tumor size was 1.7±0.8 cm in average diameter (range, 0.5–6.0 cm), and 35.5% of the tumors invaded visceral pleural. T1a, T1b, T1c and ≥T2 were observed in 47 (18.1%), 97 (37.5%), 22 (8.5%) and 93 (35.9%) patients, respectively; positive LNs were observed in 14 (5.4%) patients. The majority of patients were adenocarcinoma (245/259, 94.6%), among which 196 patients were acinar or papillary predominant.
Full table
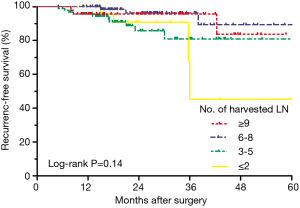
To investigate the prognostic impact of the number of harvested LN, we performed an exploratory survival analysis, using 3, 6 and 9 as the cut-offs to divide the entire population into four subgroups. Figure 2 showed that the RFS curves of patients with harvested LN ≥9 and 6–8 were close to each other, and superior than those of patients with harvested LN ≤2 and 3–5, although statistical significance was not achieved among these four groups (log-rank P=0.14). Therefore, we chose 6 harvested LN as the cut-off value for further analysis, stratifying the population as harvested LN ≥6 and <6 groups.
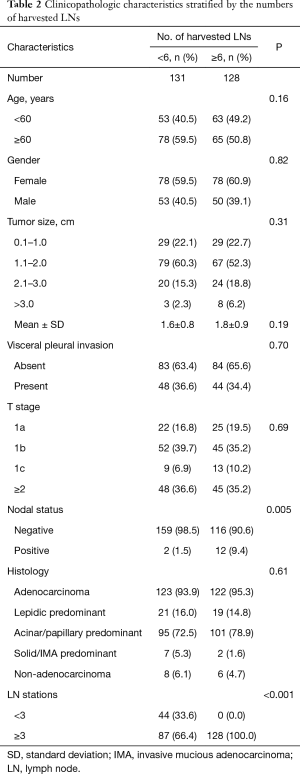
The distributions of the clinicopathologic characteristics between these two groups were summarized in Table 2. The frequency of positive nodes was 9.4% (12/128) among patients who had 6 harvested LN or more, compared with 1.5% (2/131) among those who had less than 6 harvested LN (P=0.005). Patients with harvested LN ≥6 all sampled at least three LN stations, while 66.4% (87/131) of patients with less than 6 harvested LN sampled at least three LN stations (P<0.001). Two groups had similar distributions of age, gender, tumor size, VPI, pT stage and histological subtypes (all P>0.05).
Full table
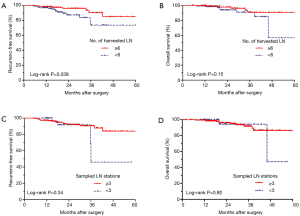
We further used Kaplan-Meier method to compare the long-term survival of two groups. The 3-year RFS of patients with harvested LN ≥6 (90.2%) was significantly higher than that of patients with harvested LN <6 (73.7%, log-rank P=0.038, Figure 3A). The multivariable Cox regression analysis was adjusted for age, gender, tumor size, VPI, pT stage, nodal status, histological subtypes and number of sampled LN station, and the results were listed in Table 3. Harvested LN ≥6 was an independent predictor of improved RFS [hazard ratio (HR) =0.35; 95% confidence interval (CI): 0.14–0.90; P=0.029]. The 3-year OS of patients with harvested LN ≥6 was 90.7%, similar to that of patients with harvested LN <6 (91.0%, log-rank P=0.15, Figure 3B). Number of harvested LN was not associated with OS on multivariable Cox analysis adjusted for covariates (HR =0.57; 95% CI: 0.17–1.88; P=0.36).

Full table
There was no significant difference in RFS (log-rank P=0.34, Figure 3C) or OS (log-rank P=0.92, Figure 3D) between sampled LN station ≥3 and <3. The number of sampled LN stations wasn’t an independent predictor for RFS (HR =0.85; 95% CI: 0.26–2.78; P=0.79) or OS (HR =0.73; 95% CI: 0.14–3.91; P=0.71) on multivariable Cox analysis.
Discussions
The latest WHO classification of lung tumors was released in 2015, and adopted the concept of AIS and MIA, which are deemed to be free from regional LN metastasis (14) and have 100% or near 100% disease-free survival after complete resection (15). Our previous study showed that intraoperative frozen section could diagnose AIS/MIA with high accuracy, so these patients are suitable candidates for sublobar resection with the assistance of frozen section (7). Radiologically pure solid tumors represent invasive pathologic features, and the long-term survival for patients with clinical stage IA lung cancer in solid nodules after sublobar resection and lobectomy are demonstrated to be comparable (6,16). A surveillance, epidemiology and end results (SEER) database analysis excluded former bronchioalveolar carcinomas, which could potentially be AIS, MIA, or invasive adenocarcinomas with lepidic components, and showed that segmentectomy led to similar survival for patient with invasive adenocarcinomas to lobectomy (17). Collectively, increasing evidence confirmed that segmentectomy could offer equivalent long-term survival for small-sized NSCLC with aggressive biological behaviors.
For some cancers, such as breast cancer and colorectal cancer, the association between the greater number of harvested LN and favorable survival has been established; thus, minimal number of dissected LNs is recommended for accurate staging (18,19). Although the current NCCN guidelines for NSCLC don’t recommend the minimal number of harvests LN, Dr. Liang et al. analyzed the US SEER data and a Chinese multi-institutional registry and found that a greater number of harvested LN was associated with more-accurate node staging and better long-term survival. Nevertheless, the appropriate extent of LN dissection of segmentectomy hasn’t been well established for the evidence is scarce. An advantage of segmentectomy over wedge resection is the more extensive LN dissection during the anatomic procedure (20). Around 10% of patients with cT1N0M0 NSCLC could develop LN metastasis (21,22). Even in primary tumors of 1 cm or smaller in diameter, N2 disease occurred in 3.8% of them (22). In the present cohort of patients undergoing either intentional or compromised segmentectomy, the LN metastasis rate was 5.4% (14/259), excluding those receiving completion lobectomies.
For patients undergoing segmentectomy who was pathologically confirmed to be node positive in postoperative permanent sections, completion lobectomy is considered to be required, but this may become quite difficult due to severe adhesion (23). Dr. Nomori and his colleagues (24) reported a case series of patients with cT1N0M0 NSCLC who were diagnosed with unanticipated pN1–2 disease after radical segmentectomy with extensive hilar and mediastinal LN dissection. Although two of five patients died during follow-up, the first recurrence sites of them were not local. This indicated that intrapulmonary and meditational LN dissection may play a role in the local control for patients undergoing segmentectomy.
We postulated that lymphadenectomy could help to reduce disease recurrence after segmentectomy for the selected patients with early-stage NSCLC. Therefore, we analyzed the data of a large cohort of patients receiving segmentectomy in our center and found that patients whose harvested LN numbers were ≥6 had significantly superior RFS. Multivariable Cox analysis showed that harvested LN ≥6 could reduce the risk of disease recurrence by 71% (HR =0.29).
These findings provide clinical evidence to support the recommendations of NCCN to sample appropriate LN stations when performing segmentectomy for patients with early-stage NSCLC. The reason of the survival benefit could be that more harvested LNs means a smaller chance of undiscovered false node-negative status and malignance remnants. In patients with pathologically confirmed T1a–2aN0M0 lung adenocarcinoma (stage I, 7th edition), LN micro-metastasis was identified in 15% of them by immunohistochemical staining for cytokeratin (AE1/AE3). Moreover, Patients with LN micro-metastasis had significantly inferior survival compared with those without micro-metastasis (25). Surgeon should obey the NCCN guidelines to sample the LN stations during segmentectomy even though the patients were clinical node-negative.
There are several limitations that should be taken into consideration. The retrospective design of the present study may introduce surgical and selection bias. Additionally, the analysis was based on a cohort of single institution with ultra-high volume and surgical expertise, limiting its generalization.
Conclusions
To the best of our knowledge, the study is currently the first to investigate the prognostic significance of lymphadenectomy for NSCLC patients undergoing segmentectomy. Our study revealed that a greater number of harvested LN was independently associated with improved RFS for NSCLC in the setting of segmentectomy.
Acknowledgements
Funding: This work was supported by the National Natural Science Foundation of China (81330056, 81472173, 81401891, 81572253, 81372525), the Key Project of Science and Technology Commission of Shanghai Municipality (JGGG1302), Shanghai Hospital Development Center (grant No. SHDC12012308), the Health and Family Planning Commission of Shanghai Municipality (grant No. 2013ZYJB0301), and the Science and Technology Commission of Shanghai Municipality (grant No. 14495810800 and 15411951601).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board [No. KS(P1733)] and written informed consent was obtained from all patients.
References
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Dziedzic R, Zurek W, Marjanski T, et al. Stage I non-small-cell lung cancer: long-term results of lobectomy versus sublobar resection from the Polish National Lung Cancer Registry. Eur J Cardiothorac Surg 2017;52:363-9. [Crossref] [PubMed]
- Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. J Clin Oncol 2014;32:2449-55. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Oncologic outcomes of segmentectomy compared with lobectomy for clinical stage IA lung adenocarcinoma: propensity score-matched analysis in a multicenter study. J Thorac Cardiovasc Surg 2013;146:358-64. [Crossref] [PubMed]
- Okada M, Yoshikawa K, Hatta T, et al. Is segmentectomy with lymph node assessment an alternative to lobectomy for non-small cell lung cancer of 2 cm or smaller? Ann Thorac Surg 2001;71:956-60; discussion 961. [Crossref] [PubMed]
- Koike T, Kitahara A, Sato S, et al. Lobectomy Versus Segmentectomy in Radiologically Pure Solid Small-Sized Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:1354-60. [Crossref] [PubMed]
- Liu S, Wang R, Zhang Y, et al. Precise Diagnosis of Intraoperative Frozen Section Is an Effective Method to Guide Resection Strategy for Peripheral Small-Sized Lung Adenocarcinoma. J Clin Oncol 2016;34:307-13. [Crossref] [PubMed]
- Sakairi Y, Yoshino I, Yoshida S, et al. Pattern of metastasis outside tumor-bearing segments in primary lung cancer: rationale for segmentectomy. Ann Thorac Surg 2014;97:1694-700. [Crossref] [PubMed]
- Rusch VW, Giroux DJ. Nodal staging in lung cancer: lymph node location or number? J Thorac Oncol 2011;6:237-8. [Crossref] [PubMed]
- Liang W, He J, Shen Y, et al. Impact of Examined Lymph Node Count on Precise Staging and Long-Term Survival of Resected Non-Small-Cell Lung Cancer: A Population Study of the US SEER Database and a Chinese Multi-Institutional Registry. J Clin Oncol 2017;35:1162-70. [Crossref] [PubMed]
- Ou SH, Zell JA. Prognostic significance of the number of lymph nodes removed at lobectomy in stage IA non-small cell lung cancer. J Thorac Oncol 2008;3:880-6. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:504-35. [Crossref] [PubMed]
- Sihoe ADL, Han B, Yang TY, et al. The Advent of Ultra-high Volume Thoracic Surgical Centers in Shanghai. World J Surg 2017;41:2758-68. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Altorki NK, Yip R, Hanaoka T, et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg 2014;147:754-62; Discussion 762-4. [Crossref] [PubMed]
- Veluswamy RR, Ezer N, Mhango G, et al. Limited Resection Versus Lobectomy for Older Patients with Early-Stage Lung Cancer: Impact of Histology. J Clin Oncol 2015;33:3447-53. [Crossref] [PubMed]
- Benson AB 3rd, Venook AP, Cederquist L, et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:370-98. [Crossref] [PubMed]
- Gradishar WJ, Anderson BO, Balassanian R, et al. Invasive Breast Cancer Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:324-54. [Crossref] [PubMed]
- Smith CB, Swanson SJ, Mhango G, et al. Survival after segmentectomy and wedge resection in stage I non-small-cell lung cancer. J Thorac Oncol 2013;8:73-8. [Crossref] [PubMed]
- Medbery RL, Gillespie TW, Liu Y, et al. Nodal Upstaging Is More Common with Thoracotomy than with VATS During Lobectomy for Early-Stage Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol 2016;11:222-33. [Crossref] [PubMed]
- Zhang Y, Sun Y, Shen L, et al. Predictive factors of lymph node status in small peripheral non-small cell lung cancers: tumor histology is more reliable. Ann Surg Oncol 2013;20:1949-54. [Crossref] [PubMed]
- Omasa M, Date H, Takamochi K, et al. Completion lobectomy after radical segmentectomy for pulmonary malignancies. Asian Cardiovasc Thorac Ann 2016;24:450-4. [Crossref] [PubMed]
- Nomori H, Mori T, Izumi Y, et al. Is completion lobectomy merited for unanticipated nodal metastases after radical segmentectomy for cT1 N0 M0/pN1-2 non-small cell lung cancer? J Thorac Cardiovasc Surg 2012;143:820-4. [Crossref] [PubMed]
- Dai C, Xie H, Kadeer X, et al. Relationship of Lymph Node Micrometastasis and Micropapillary Component and Their Joint Influence on Prognosis of Patients with Stage I Lung Adenocarcinoma. Am J Surg Pathol 2017;41:1212-20. [Crossref] [PubMed]


