Retrospective study on video-assisted vs. open mediastinal lymphadenectomy for non-small cell lung cancer: a propensity-matched analysis
Introduction
Since the first case of performing anatomical lobectomy and systemic mediastinal lymph node (MLN) dissection under video-assisted thoracoscopic surgery (VATS) was reported in 1990s, treating VATS procedure has been rapidly popularized for its minor surgical trauma, rapid postoperative recovery, short length of stay, good patient tolerance and so on (1-5). More importantly, its surgical safety and effectiveness have been extensively verified. The National Comprehensive Cancer Network (NCCN) has suggested in its non-small cell lung cancer (NSCLC) Treatment Guideline in 2012 that, VATS can be used as a standard procedure for treating early NSCLC (6).
During the past decade has witnessed the rapid development of VATS technology, thanks to the innovations of surgical technique and sophisticated instruments. The surgical method has developed from the traditional tri- and quadric-port VATS to single intercostal or even uniport approach. In addition, the surgical method has steadily evolved from wedge resection or pulmonary lobectomy to anatomical segmentectomy, sleeve pulmonary lobectomy and even combined lobectomy (7-9). However, regardless of the development of surgical method and indication, the surgical outcomes of VATS are always the most attentive focus, while the MLN dissection is evaluation criterion of lung cancer surgery.
In this context, a total of 497 NSCLC patients underwent pulmonary lobectomy or systemic MLN dissection were retrospectively studied in this research. The baseline data of samples were matched at a ratio of 1:1 using the propensity score matching (PSM) method. Thus, differences in effectiveness and risk of surgery between VATS and thoracotomy were compared and analyzed.
Methods
Patients
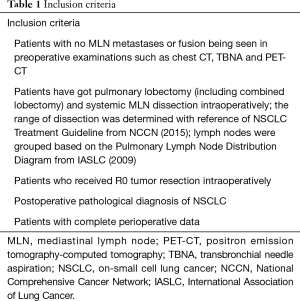
Clinical data of 497 NSCLC patients who underwent lobectomy and systemic MLN dissection in our hospital from January 2010 to July 2015 were analyzed, and the inclusion criteria are shown in Table 1. The patients were divided into VATS group (n=242) and traditional thoracotomy (TT) group (n=255) according to patients’ intentions. Comparison of preoperative clinical data include age, sex, smoking history, forced expiratory volume (FEV1%), maximum ventilatory volume (MVV%), hypertension, chronic obstructive pulmonary disease (COPD), diabetes, coronary heart disease, tumor site, tumor size, and preoperative MLN stage. MLNs were grouped in accordance with the Pulmonary Lymph Node Distribution Diagram from (International Association of Lung Cancer) IASLC [2009] (10). The range of MLN dissection was determined with reference to NSCLC Treatment Guideline from NCCN (2015) (11).
Full table
Surgical procedures
All surgeries received operations under general anesthesia with one-lung ventilation by clinicians who had extensive experience in thoracoscopic surgery.
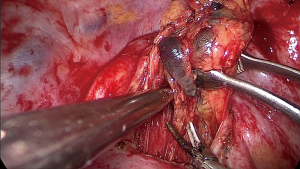
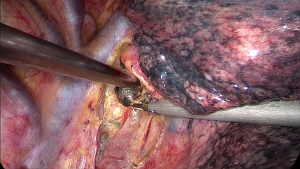
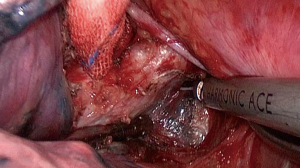
During the surgery the patient took the supine position. The surgeon was at the ventral side of patients and the assistant at the dorsal side. The VATS group adopted traditional three-hole thoracoscopic techniques. The camera hole was 1cm, located at 2 cm behind the posterior axillary line between the 8th ribs. The primary operating hole was 3–4 cm located between the midaxillary line and anterior axillary line was on the intercostal of the 4th rib. For lower lung surgery it can be placed at the 5th rib. And the second operating incision was only 5 mm located 2 cm in front of the scapular line. The TT group had the standard posterior-lateral or muscle sparing thoracotomy (MST). Systemic MLN dissection was executed after the pulmonary resection and lung cancer had been confirmed by intraoperative pathological examination (Figures 1-3). The surgeon or assistant recorded the operation time, intraoperative blood loss, secondary injury and grouped the dissected lymph nodes before they were sent for the biopsy.
Statistical analysis
Research data in retrospective studies are frequently associated with distinct selection bias. PSM is a statistical method extensively applied in retrospective studies in recent years. It can reduce the sample selection bias thus make the results more scientific and reliable (12).
All statistical analyses were performed with SPSS (version 19.0). The measurement data was expressed as incidence rates and 95% confidence intervals (CIs). The Kolmogorov-Smirnov test indicated that data of each variable fitted normal distribution. Counting data differences between the two groups were analyzed by U test, while binomial test was used when the date conformed to binomial distribution. P value <0.05 was deemed statistically significant for all analyses.
Results
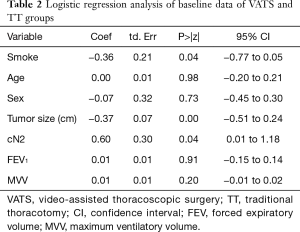
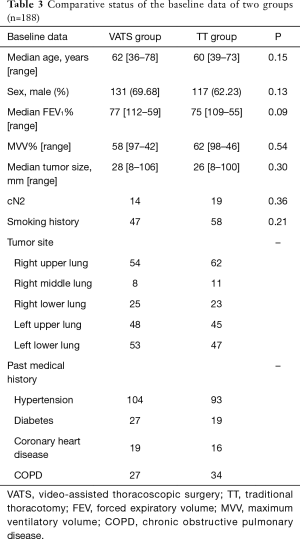
Logistic regression analysis of baseline data between two primary groups are shown in Table 2, which suggested statistically significant differences in smoking history, tumor size and preoperative MLN stage between two groups (P<0.05). After stratification matching by selecting patients underwent VATS and looking for a control patient at a ratio of 1:1 according to these three variables, 376 cases were matched for PSM finally (P>0.05, Table 3).
Full table
Full table
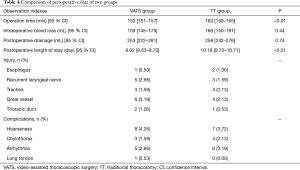
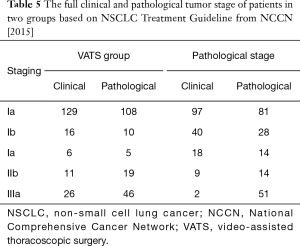
No surgery-related death case was reported in both groups during hospitalization. Total 11 cases had (4 were in VATS group, 7 in the TT group) more than 800 mL of drainage within 24 hours after surgery. One patient in the TT group underwent a re-operation for hemostasis. Surgery time and postoperative length of stay in the VATS group were remarkably shortened with the comparison of TT group (P<0.01, Table 4). The intraoperative blood loss, intraoperative tracheal, esophageal, recurrent laryngeal nerve, thoracic duct and great vascular injury, drainage volume 24 h after surgery between two groups were not statistically significant (P>0.05). Besides, the postoperative complications e.g., hoarseness, arrhythmia and chylothorax between two groups also were not statistically significant (P>0.05, Table 4). The full tumor stage of patients is shown in Table 5.
Full table
Full table
In this study the independent grouping was not applicable due to the MLN grouping pattern (2R and 4R, 5 and 6, as well as groups 8 and 9). In terms of the ability of MLN dissection in two groups, the group number of MLN in VATS group was higher than that in TT group with statistically significant (P<0.01). The total number of dissected MLN in VATS group was slightly lower than that in TT group, but the difference was not statistically significant (P>0.05). Number of dissected MLN among the groups 2+4, 7 and 8+9 in the right, as well as in 5+6 and 8+9 in the left between two groups were not statistically significant (P>0.05). However, the number of MLN in group 7L among VATS group was lower than that in TT group, with a statistical significance (P<0.01). The overall metastatic rate in VATS group was slightly lower than that in TT group, but the difference was not statistically significant (P>0.05). Data comparison is illustrated in Table 6.

Full table
Discussion
Thoroughness of MLN dissection is one of the most important criterions for evaluating the effectiveness of lung cancer surgery. The surgical methods include systemic MLN dissection, systemic MLN sampling, and selective MLN dissection. None of these methods has been proved as the optimal approach yet. However, most research data have supported systemic MLN dissection as a better choice for two main reasons. Firstly, preoperative non-invasive examinations, such as enhanced CT or positron emission tomography-computed tomography (PET-CT), display poor specificity in judging MLN metastasis, which is associated with certain ratio of cN0-pN2 phenomenon (13,14). Secondly, it can promote a more rigorous outcome of postoperative TNM staging, so as to instruct whether further therapy such as chemotherapy should be given to the patients.
Pulmonary lobectomy under VATS has become a recommended procedure by NCCN to treat early NSCLC. Its clinical application has been increasingly expanded with the progressively matured techniques. VATS has been developed quickly from anatomical segmentectomy to radical resection for advanced lung cancer and even to complicated surgical fields like, surgery after neoadjuvant chemotherapy, sleeve resection of pulmonary artery and bronchus, as well as total pneumonectomy for treating central lung cancer (9,15,16). Nonetheless, regardless of the distinct advantages and changes in indication, surgical effectiveness remains the current focus of attention. This is particularly true for advanced NSCLC patients.
Palade et al. had carried out a prospective randomized controlled study on the effectiveness of MLN dissection under VATS (17). The results indicated that operation time was distinctly shorter in VATS group, with the difference being statistically significant (P<0.01). However, differences in dissected MLN numbers and group numbers, as well as perioperative complications between two groups were not statistically significant (P>0.05). The effectiveness of VATS for advanced lung cancer has also been reported in recent years. Shao et al. had carried out a follow-up study on 51 patients with clinical stage IIIA NSCLC underwent VATS (18). The results revealed that the average MLN numbers and group numbers were 22.5 and 5.0, respectively, and no one was converted into thoracotomy. All these studies have demonstrated that VATS is equally effective to thoracotomy for lung cancer.
Similar conclusion has been drawn in this study. To be specific, MLN numbers between two groups is not statistically significant (P=0.12). However, the average group numbers are higher in VATS group than in TT group, with the difference being of statistical significance (P<0.01). Further stratification research reveals that differences in MLN dissection ability among all groups in right-sided lung cancer surgery between two groups are not statistically significant (P>0.05). But the MLN numbers of group 7 in VATS group is notably lower than that in TT group, with the difference being statistically significant (P<0.01). Two assumptions can possibly be used to explain this result. Firstly, the left carina has special and complicated anatomical location, which has added a certain difficulty in sufficient exposure of esophagus and carina under endoscope relative to that in the right side. Secondly, dissection of group 7 lymph nodes in the left is associated with relatively high incidence of broncho-arterial hemorrhage because of unclear local anatomy. Therefore, it is likely to induce accessory injury or insufficient dissection. In fact, bronchial or esophageal injury cases have ever happened during VATS in this study, which had markedly extended the operation time. Nagashima et al. had reported their surgical experience of dissection through the posterior approach under VATS, which has a certain value of reference (19). Recently, Baste et al. had reported the feasibility and technique points of anterior approach surgery (20). These procedures may facilitate the dissection of group 7 lymph nodes.
Nonetheless, this study is inevitably associated with certain drawbacks. Firstly, with the accumulation of clinical experience and improvement of surgical instruments, the operation time, blood loss, postoperative drainage and postoperative length of stay is always improving in our study. This will lead to a difference in the research results. In order to limit the deviations, we have excluded all cases in the initial phase by the time we begin to perform VATS before 2010. Secondly, some cases for submission in both groups have combined groups 2R and 4R, 5 and 6, as well as 8 and 9, which make it very difficult for grouping statistics. Meanwhile, we can hardly assure that lymph nodes in groups 3A, 3P and 4L can be completely dissected in all patients due to the anatomical location and physiological factors. Therefore, these three groups are not included. Thirdly, lymph node can easily be broken into small pieces during the surgery, making it harder to figure out the exact number of dissected MLN.
Conclusions
Our study proved MLN dissection and pulmonary lobectomy under VATS has the similar surgical effectiveness to thoracotomy with quicker recovery, less postoperative complication and length of hospital stay. Besides, it also suggests thoracotomy has an advantage on the dissection the MLN in group 7L. This shortcoming of VATS may be overcome via the improvement of operative technique, but it warrants further validation.
Acknowledgements
Funding: This work was supported by grants from the Science and Technology Department of Zhejiang Province (2013c03044-7, YC).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of Second Affiliated Hospital of Zhejiang University School of Medicine (No. 2014-032) and written informed consent was obtained from all patients.
References
- McKenna RJ Jr. Lobectomy by video-assisted thoracic surgery with mediastinal node sampling for lung cancer. J Thorac Cardiovasc Surg 1994;107:879-81; discussion 881-2. [PubMed]
- Liu K, Zhao J, Zhang W, et al. Video-assisted thoracoscopic surgery for non-small-cell lung cancer in elderly patients: a single-center, case-matched study. Int J Clin Exp Med 2015;8:11738-45. [PubMed]
- Rizk NP, Ghanie A, Hsu M, et al. A prospective trial comparing pain and quality of life measures after anatomic lung resection using thoracoscopy or thoracotomy. Ann Thorac Surg 2014;98:1160-6. [Crossref] [PubMed]
- Whitson BA, D'Cunha J, Andrade RS, et al. Thoracoscopic versus thoracotomy approaches to lobectomy: differential impairment of cellular immunity. Ann Thorac Surg 2008;86:1735-44. [Crossref] [PubMed]
- Papiashvilli M, Stav D, Cyjon A, et al. Lobectomy for non-small cell lung cancer: differences in morbidity and mortality between thoracotomy and thoracoscopy. Innovations (Phila) 2012;7:15-22. [Crossref] [PubMed]
- Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer. J Natl Compr Canc Netw 2012;10:1236-71. [Crossref] [PubMed]
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Swanson SJ. Segmentectomy for lung cancer. Semin Thorac Cardiovasc Surg 2010;22:244-9. [Crossref] [PubMed]
- Mahtabifard A, Fuller CB, McKenna RJ Jr, et al. Video-assisted thoracic surgery sleeve lobectomy: a case series. Ann Thorac Surg 2008;85:S729-32. [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Ettinger DS, Wood DE, Akerley W, et al. Non-Small Cell Lung Cancer, Version 6. 2015. J Natl Compr Canc Netw 2015;13:515-24. [Crossref] [PubMed]
- Dehejia R, Wahba S. Propensity score-matching methods for nonexperimental causal studies. Rev Econ Stat 2002;84:151-61. [Crossref]
- Lee AY, Choi SJ, Jung KP, et al. Characteristics of Metastatic Mediastinal Lymph Nodes of Non-Small Cell Lung Cancer on Preoperative F-18 FDG PET/CT. Nucl Med Mol Imaging 2014;48:41-6. [Crossref] [PubMed]
- Ozawa Y, Hara M, Sakurai K, et al. Diagnostic accuracy of (18)F-2-deoxy-fluoro-D-glucose positron emission tomography for pN2 lymph nodes in patients with lung cancer. Acta Radiol 2010;51:150-5. [Crossref] [PubMed]
- Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. J Clin Oncol 2014;32:2449-55. [Crossref] [PubMed]
- Ma L, Liu C, Liu L. Video-assisted thoracoscopic surgery right upper posterior segmentectomywith systemic mediastinal lymph node dissection. J Thorac Dis 2014;6:1819-21. [PubMed]
- Palade E, Passlick B, Osei-Agyemang T, et al. Video-assisted vs open mediastinal lymphadenectomy for Stage I non-small-cell lung cancer: results of a prospective randomized trial. Eur J Cardiothorac Surg 2013;44:244-9; discussion 249. [Crossref] [PubMed]
- Shao W, Liu J, Liang W, et al. Safety and feasibility of video-assisted thoracoscopic surgery for stage IIIA lung cancer. Chin J Cancer Res 2014;26:418-22. [PubMed]
- Nagashima T. Thoracoscopic left mediastinal lymph node dissection. Ann Transl Med 2016;4:10. [PubMed]
- Baste JM, Haddad L, Melki J, et al. Anterior subcarinal node dissection on the left side using video thoracoscopy: an easier technique. Ann Thorac Surg 2015;99:e99-101. [Crossref] [PubMed]


