Electromagnetic navigation bronchoscopy—Chungnam National University Hospital experience
Introduction
Small pulmonary nodules that need to be resected for diagnostic or therapeutic purpose are increasing. The computed tomography (CT) screening of the chest for high risk patients of lung cancer allows more early detection of a lung cancer (1,2). To find small pulmonary nodules or ground grass nodules (GGNs) with video-assisted thoracoscopic surgery (VATS) is very challenging (3-5). Previous several studies have reported that it is very difficult to localize a non-palpable small diameter and/or deeply located nodules from the visceral pleural margin during minimally invasive thoracoscopic surgery (5). Several conventional methods to localize small nodules or GGNs are used in the surgical field. Most of these procedures use the fluoroscopic-guided or CT-guided transthoracic approaches with dye marking, coil embolization, or hook-wire (6-12). These methods require additional radiation exposure, and may cause some complications such as pneumothorax or hemothorax. More importantly, the procedure-related complications lower the accuracy of localization. The endobronchial ultrasound-guided transbronchial needle aspiration is not available for peripheral lung lesions.
Nowadays, newly established method has promised to identify the small pulmonary nodules or GGNs through the electromagnetic navigation bronchoscopy (ENB)-guided transbronchial dye-marking (5,13-15). The effectiveness of the ENB procedure for a diagnostic biopsy has been reported in several studies (16-19). The ENB-guided transbronchial needle aspiration or biopsy showed a higher accuracy and lower complication rates, such as a pneumothorax or hemothorax, than the conventional percutaneous core needle biopsy. More recently, the ENB procedure is increasingly accepted as a valuable tool in thoracic surgery. A few researchers have reported that the ENB-guided localization can be of help to resect the pulmonary lesion (5,13-15). In this study, we aimed to evaluate how well the ENB-guided dye-marking is seen in the operation field, how well the marking localizes the pulmonary lesion, and whether the pulmonary lesion was resected sufficiently utilizing this procedure.
Methods
We retrospectively reviewed medical records from prospectively collected database of the patients who underwent an ENB procedure for a biopsy and/or localization of a pulmonary resection at the Chungnam National University Hospital from January 2017 to January 2018. The Institutional Review Board of the Chungnam National University Hospital approved this retrospective study.
ENB, dye marking, and surgical procedure
The patients re-checked a non-enhance chest CT according to recommended protocol of the company (Medtronic, Minneapolis, MN), to reconstruct the virtual bronchoscopy on the preoperative day. Two surgeons (M-W.K. and H.J.C) performed planning, ENB-guided dye marking, and VATS surgery. All procedures from planning to the surgery for each patient were performed by the operating surgeon in the operating room at the time of the resection. We set the target and planned the pathway to the target using the superDimensionTM navigation system and planning software (Medtronic, Minneapolis, MN). Initially, we tried to target directly the nodule, but later we routinely set up the several targets including direct and indirect targets for one lesion and the several pathways per target. If access to first target was not possible despite attempts through several pathways during ENB-procedure, we moved to the next target. If the dye was injected to indirect target, we considered the positional relationship between the indirect target and the actual nodule based on the chest CT to localize the nodule.
The patients underwent general anesthesia with a single-lumen intubation in operating room. Subsequently, each target was marked by indigo carmine dye using extended working catheter under locatable electromagnetic guide. After the target was located, the locatable guide was removed, and a 25-gauge needle preloaded with indigo carmine was inserted through the working catheter. We did not use fluoroscopy or CT to verify the deployment of the needle, and just inserted the needle catheter in the same length as the length of the electromagnetic guide after the location to the target. After the deployment of the bronchoscopic needle, the indigo carmine 0.5 mL was injected. Then, a needle catheter was removed and we confirmed the location of the catheter by re-insertion of the electromagnetic guide through the working catheter. After sufficient localization, the endotracheal tube was replaced with a double-lumen endotracheal tube. In the case of a single port VATS according to preoperative plan, we used a 15 mm single port for thoracoscope and instruments. If the dye was visualized on the inspection of lung and the location of stained lung was proper to anchor with the suture, a single port VATS surgery was performed (Tower crane technique) (16). If not, we changed to a conventional three-port VATS surgery. For depth of resection, we referred to the CT assessment, and the resection margin was confirmed by gross finding of cross section or frozen-section analysis of the specimen.
ENB-guided biopsy was performed using the superDimensionTM Triple needle brush (Medtronic, Minneapolis, MN) and/or the biopsy forceps for bronchoscope. The planning and the procedure were same to ENB-guided localization, and the lesions were targeted directly. After the target was located, the locatable guide was removed, and the samples were obtained by the Triple needle brush or the biopsy forceps through the working catheter. The samples were sent to the pathology department for rapid on-site evaluation (ROSE) or frozen section. If the finding was negative for malignancy, we did re-biopsy and ended the procedure.
Results
Patient characteristics
A total of 37 ENB-guided dye-markings or biopsies for 37 lesions in 30 patients were performed. The males were 20 in number, and the median age was 64 years old. The characteristics of the patients are listed in Table 1. Thirty-two ENB-guided localizations using dye-marking were performed in 25 patients, and 5 ENB-guided biopsies were performed in the other patients.
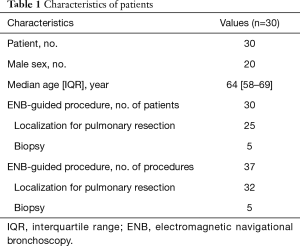
Full table
Characteristics of the pulmonary lesions for localization and surgery
An ENB-guided localization was performed with dye-marking using indigo carmine, and we estimated a visualization of the dye-marking on the lung surface in the operating field. In a total of 32 ENB-guided localizations, we did not find the dye-marking or discoloration in 4 localizations (12.5%). The characteristics of the pulmonary lesions were described in Table 2. The median value of the nodule size was 9 mm (IQR: 7–13 mm), and the median value of the distance from the pleura was 6 mm (IQR: 3–10 mm). There are no fatal complications related to the ENB-guided dye marking, but there were two patients who had an adverse event. Mild bronchial bleeding was occurred during the ENB procedure, but the bleeding spontaneously stopped. The most common lobar location was the right lower lobe (11 cases, 34.4%), and all cases of the localization failure were at the right lower lobe. There were four cases of failed localization that was not helpful in locating the nodule because the stain was invisible or indistinguishable on lung surface (Table 3). In this case, an indigo carmine was injected to the chest wall in the sixth case of pulmonary localization, deep parenchyme in 8th case, and in a pleural cavity in 19th case. In the 10th case, we did not distinguish the dye-staining from anthraco pigmentation because of a severe pulmonary anthracosis. There was no operative mortality (0%). A pathologic diagnosis was obtained from surgically resected specimen (not included ENB biopsy: 32 of 32 localizations, 100%), neoplastic lesions were 23 cases (72%). In all of the cases, a primary lung cancer and a metastatic lung cancer were identified in 11 cases and 11 cases, respectively. The other cases were noted as benign lesions (Table 4). In these cases, all margins of the nodules were negative.
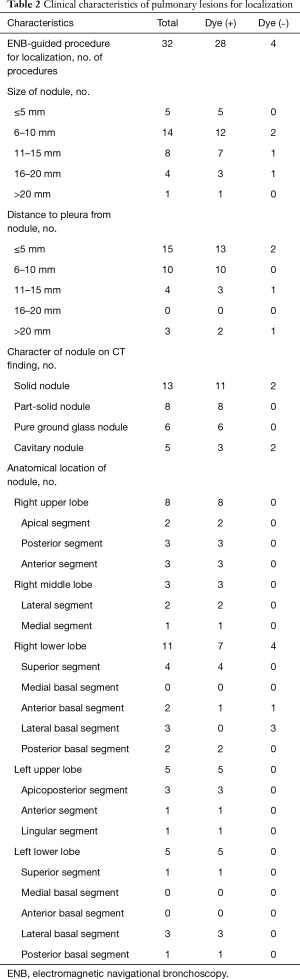
Full table
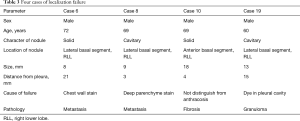
Full table
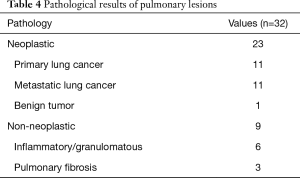
Full table
Comparisons of single port VATS and conventional three-port VATS
We routinely used conventional three-port VATS to resect the lesion, but if the location of the peripheral solid nodule was appropriate to anchor the suture and the stain was visualized on lung inspection, we tried to resect using a 15 mm single port with an anchoring suture. Eleven patients underwent single port VATS, and the remaining 21 patients conventional three-port VATS (Table 5). The nodule size between the two groups was not significantly different. The distance from the pleura was 4 mm in a single port VATS group and 7 mm in a conventional VATS group, but the difference between the two groups was not shown. In comparison of pathology between the two groups, the primary lung cancer was significantly lower in the single port VATS group, than in the conventional three-port VATS group. However, an additional resection due to the inappropriate resection margin was only noted as one case in the conventional VATS group.
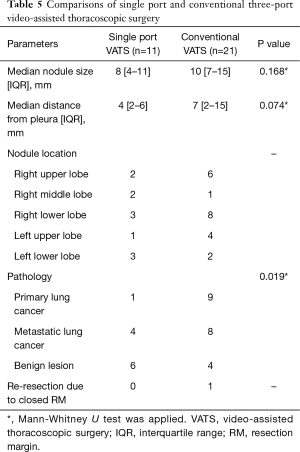
Full table
ENB-guided biopsy
Five ENB-guided biopsies were performed in five patients. Three patients had lung or other malignancy and underwent the procedure for tissue diagnosis of metastasis or recurrence. Two patients were referred for the evaluation of solitary pulmonary nodule in location where it is difficult to perform the percutaneous core-needle biopsy. The size of the lesions was 9, 13, 30, 30, and 40 mm, respectively. Three patients were identified as the malignancy. One patient was benign lesion and the lesion was stable in 1-year followed chest CT. The other patient was also benign lesion on ENB-guided biopsy, but three months later, was confirmed as malignancy by percutaneous core-needle biopsy. There was no complication except minor bronchial bleeding.
Discussion
The incidence of small nodules that need to have identifying pathology or early stage lung cancers present as GGN are thus increasing (1,2). The lesion that is difficult to perform a percutaneous conventional method like core-needle biopsy for diagnosis which is requiring surgical resection and the demand for a sublobar resection is increasing in the early stage of lung cancer, such as a small GGN (5). An accurate localization is very important for these sublobar resection or non-anatomical resection. Several conventional methods have been performed to localize for small nodule, but these methods require additional radiation and other specialists (6-12). Also some complications such as a pneumothorax or a hemothorax reduce the accuracy of the procedure. An ENB-guided procedure has already been applied in the diagnostic field (16-19), but only recently performed in the surgical field (5,13-15). This procedure is accomplished through a transbronchial approach; therefore, it has a lower complication rate than the pre-existing transthoracic approach. In our data, just two patients showed mild intrabronchial bleeding, which stopped spontaneously. There was no hemothorax. However, we could not estimate for the occurrence of pneumothorax because we did not use a fluoroscopy or a CT during the procedure and the thoracic surgery was performed continuously after the procedure.
The nodule size 10 mm or less than 10 mm was the most common in this study and all nodules were resected with negative resection margin. This means that small size nodules even though less than 10 mm can be detected by an ENB-guided localization and resected with minimally invasive thoracoscopic surgery. The most common distance from the visceral pleura to lesion was less than 5 mm. In this range, two localizations were failed. Initially, we directly targeted the peripheral nodule or peripherally targeted as possible for the visualization of staining. The peripheral injecting of the dye had a risk to penetrate visceral pleura and, in case of penetration of visceral pleura, we found the dye staining of the chest wall or the dye collection in the pleural cavity. Of course, in this scenario, we had the methods available to localize the nodule. The methods included, first, to re-mark manually of the lung region to face the stained region of the chest wall after lung inflation, and second, to find the penetration point through air bubbling with saline spreading with two lung ventilation.
The failure of the ENB-guided localization was noted in 4 cases (12.5%). This failure rate was slightly higher than other conventional methods (6-8,10,11). In our study, three failure cases were occurred within the initial 3 months. After a learning period of 3 months (10 cases for two surgeons), just 1 case of 21 localizations was failed. After our initial experiences, we learned which position of the electromagnetic locatable guide tip is appropriate and how to control depth of catheter for the dye injection. We were able to reduce the localization failure due to the visceral pleura penetration by placing the electromagnetic locatable guide tip at a distance of 10 mm from the visceral pleura considering a length of dye injecting needle or by injecting dye at several depth levels. The nodules to fail for localization were all located in the right lower lobe, especially the lateral basal segment, although this segment was easy to approach the lesion by the ENB-guided procedure. However, we thought that the relation of anatomical location of the nodule and the failed localization is not meaningful because the cause of the localization failure was different in each case.
The most common pathology was primary lung cancer and metastatic lung cancer. All of the primary lung cancer was adenocarcinoma. In the sublobar resection of an early stage lung cancer, it is very important to obtain a sufficient resection margin (20,21). In our study, all patients obtained a negative resection margin, but we resected the lung under the uncertainty of a deep resection margin. In order to secure the deep resection margin, the methods to visualize the localization in three-dimensions are needed. The localization by placement of a fiducial marker through ENB-guided procedure may be helpful to ensure the deep resection margin (22-24), but additional radiation exposure is required under fluoroscopy. The use of a near-infrared fluorescence imaging, with an ENB-guided indocyanine green injection is also expected to be helpful to secure the deep resection margin without radiation exposure (25,26).
Ng et al. (27) reported that the single port VATS is possible for non-palpable pulmonary nodule under localization with hookwire in hybrid operating room. We don’t have a hybrid operating room, so we could not use a hookwire or a fiducial marker. We tried to do the single port VATS in the super-selected case of a small peripheral solid nodule and well-stained localization. Total 11 localizations were resected by a single port VATS surgery. The median nodule size and median distance from the pleura were smaller than conventional three-port VATS surgery by our intended selection, but the differences were not significant. A benign lesion was significantly more in the single port VATS group than the conventional VATS group. There was no re-resection in the single port VATS group. This means that the single port VATS with an ENB-guided pulmonary localization can achieve a complete resection in a properly selected case.
In conclusion, this study supposes that the ENB-guided dye localization by a well-trained thoracic surgeon enables the accurate intraoperative identification of a GGN or small pulmonary nodule with minimal complications, and makes minimally invasive surgery possible even for nodules that were difficult to localize. This study need to be proven by large-scale studies and thoracic surgeons should participate for further investigation of this method.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Institutional Review Board of the Chungnam National University Hospital approved this retrospective study.
References
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Aberle DR, DeMello S, Berg CD, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med 2013;369:920-31. [Crossref] [PubMed]
- Suzuki K, Nagai K, Yoshida J, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest 1999;115:563-8. [Crossref] [PubMed]
- Saito H, Minamiya Y, Matsuzaki I, et al. Indications for preoperative localization of small peripheral pulmonary nodules in thoracoscopic surgery. J Thorac Cardiovasc Surg 2002;124:1198-202. [Crossref] [PubMed]
- Awais O, Reidy MR, Mehta K, et al. Electromagnetic navigation bronchoscopy-guided dye marking for thoracoscopic resection of pulmonary nodules. Ann Thorac Surg 2016;102:223-9. [Crossref] [PubMed]
- Lizza N, Eucher P, Haxhe JP, et al. Thoracoscopic resection of pulmonary nodules after computed tomographic-guided coil labeling. Ann Thorac Surg 2001;71:986-8. [Crossref] [PubMed]
- Lin MW, Tseng YH, Lee YF, et al. Computed tomography-guided patent blue vital dye localization of pulmonary nodules in uniportal thoracoscopy. J Thorac Cardiovasc Surg 2016;152:535-44.e2. [Crossref] [PubMed]
- Chen S, Zhou J, Zhang J, et al. Video-assisted thoracoscopic solitary pulmonary nodule resection after CT-guided hookwire localization: 43 cases report and literature review. Surg Endosc 2011;25:1723-9. [Crossref] [PubMed]
- Doo KW, Yong HS, Kim HK, et al. Needlescopic resection of small and superficial pulmonary nodule after computed tomographic fluoroscopy-guided dual localization with radiotracer and hookwire. Ann Surg Oncol 2015;22:331-7. [Crossref] [PubMed]
- Grogan EL, Jones DR, Kozower BD, et al. Identification of small lung nodules: technique of radiotracer-guided thoracoscopic biopsy. Ann Thorac Surg 2008;85:S772-7. [Crossref] [PubMed]
- Sancheti MS, Lee R, Ahmed SU, et al. Percutaneous fiducial localization for thoracoscopic wedge resection of small pulmonary nodules. Ann Thorac Surg 2014;97:1914-8. [Crossref] [PubMed]
- Wicky S, Mayor B, Cuttat JF, et al. CT-guided localizations of pulmonary nodules with methylene blue injections for thoracoscopic resections. Chest 1994;106:1326-8. [Crossref] [PubMed]
- Marino KA, Marino MD, Jennifer L, et al. Electromagnetic navigation bronchoscopy for identifying lung nodules for thoracoscopic resection. Ann Thorac Surg 2016;102:454-7. [Crossref] [PubMed]
- Muñoz-Largacha JA, Ebright MI, Litle VR, et al. Electromagnetic navigational bronchoscopy with dye marking for identification of small peripheral nodules during minimally invasive surgical resection. J Thorac Dis 2017;9:802-8. [Crossref] [PubMed]
- Pearlstein DP, Quinn CC, Burtis CC, et al. Electromagnetic navigation bronchoscopy performed by thoracic surgeons; one center’s early success. Ann Thorac Surg 2012;93:944-9. [Crossref] [PubMed]
- Chong Y, Cho HJ, Kang SK, et al. Outcomes of the Tower Crane Technique with a 15-mm Trocar in Primary Spontaneous Pneumothorax. Korean J Thorac Cardiovasc Surg 2016;49:80-4. [Crossref] [PubMed]
- Brownback KR, Quijano F, Latham HE, et al. Electromagnetic navigational bronchoscopy in the diagnosis of lung lesions. J Bronchology Interv Pulmonol 2012;19:91-7. [Crossref] [PubMed]
- Eberhardt R, Anantham D, Herth F, et al. Electromagnetic navigation diagnostic bronchoscopy in peripheral lung lesions. Chest 2007;131:1800-5. [Crossref] [PubMed]
- Gildea TR, Mazzone PJ, Karnak D, et al. Electromagnetic navigation diagnostic bronchoscopy: a prospective study. Am J Respir Crit Care Med 2006;174:982-9. [Crossref] [PubMed]
- Leong S, Ju H, Marshall H, et al. Electromagnetic navigation bronchoscopy: A descriptive analysis. J Thorac Dis 2012;4:173-85. [PubMed]
- Mohiuddin K, Haneuse S, Sofer T, et al. Relationship between margin distance and local recurrence among patients undergoing wedge resection for small (</=2cm) non-small cell lung cancer. J Thorac Cardiovasc Surg 2014;147:1169-75. [Crossref] [PubMed]
- Sawabata N, Ohta M, Matsumura A, et al. Optimal distance of malignant negative margin in excision of nonsmall cell lung cancer: A multicenter prospective study. Ann Thorac Surg 2004;77:415-20. [Crossref] [PubMed]
- Keating J, Singhal S. Novel methods of intraoperative localization and margin assessment of pulmonary nodules. Semin Thorac Cardiovasc Surg 2016;28:127-36. [Crossref] [PubMed]
- Anantham D, Feller-Kopman D, Shanmugham LN, et al. Electromagnetic navigation bronchoscopy-guided fiducial placement for robotic stereotactic radiosurgery of lung tumors: a feasibility study. Chest 2007;132:930-5. [Crossref] [PubMed]
- Zhao ZR, Lau RW, Ng CS. Hybrid theatre and alternative localization techniques in conventional and single-port video-assisted thoracoscopic surgery. J Thorac Dis 2016;8:S319-27. [PubMed]
- Anayama T, Qiu J, Chan H, et al. Localization of pulmonary nodules using navigation bronchoscope and a near-infrared fluorescence thoracoscope. Ann Thorac Surg 2015;99:224-30. [Crossref] [PubMed]
- Ng CS, Man Chu C, Kwok MW, et al. Hybrid DynaCT scan-guided localization single-port lobectomy. Chest 2015;147:e76-8. [Crossref] [PubMed]


