Imaging techniques for minimally invasive thoracic surgery—Korea University Guro Hospital experiences
Introduction
Image-guided surgery can be defined as surgery where the operator utilizes surgical devices that incorporate the use of tracking technology in conjunction with a fusion of images in order to guide surgical procedures (1). This method mainly uses video cameras or electromagnetic fields to capture the anatomical information of the patients, and the surgeon’s movement in relation to the patient, and subsequently relay such information to computer monitors in the operating room. Image-guided surgery was originally developed for brain surgery (using stereotactic devices) or radiosurgery that are generally guided by multiple imaging modalities, such as computed tomography (CT), magnetic resonance imaging, and positron emission tomography (PET) (2).
With recent advances in video-assisted thoracoscopic surgery (VATS), there has been an increasing use of imaging technology to facilitate minimal resection with minimal incision (3,4). Generally, for the diagnosis of indeterminate pulmonary lesions, CT-guided transthoracic needle biopsy of small lung lesions is performed (5,6). However, this technique might be technically limited and problematic because of low diagnostic yield for small ground-glass lesions (7,8). Thus, VATS is a safe and minimally invasive diagnostic procedure in such cases, which can also be used simultaneously for the definite treatment of indeterminate pulmonary lesions (9,10).
Imaging modalities for thoracic surgery include techniques, such as intraoperative ultrasonography (11,12) and use of fluorescent dyes (13,14) to identify small nonpalpable nodules or to visualize sufficient resection margins during sublobar resection (15). They also involve preoperative localization techniques, such as dye injection (16,17), radioisotope administration (18,19), or metallic device use (microcoils or hook wires) with CT (20,21) or electromagnetic navigational bronchoscopy (ENB) (22,23). Historically, the CT-guided hook-wire marking method is the most commonly used technique. The techniques using microcoils or radiocontrast materials, such as lipiodol, also showed acceptable results in several studies (15); however, the debate for establishing an ideal localization technique for VATS-based resection is currently ongoing as is the development of noninvasive techniques using fluorescent dyes or intraoperative ENB. In this article, we described our experiences with various types of image-guided thoracic surgeries and reported the outcomes. We also discuss our recent preliminary investigation of ENB-based localization for VATS and robotic surgery.
Dual localization techniques using radiocontrast and hook wires
Preoperative hook-wire marking via CT-guided localization is the most commonly used techniques for VATS-based resection of small peripheral pulmonary nodules. The hook-wire technique has been reported to be accurate and is associated with high success rates, while resulting in higher rates of pneumothorax, bleeding, and dislodgement of hook-wires compared to other methods (24,25). Wire dislodgement or migration might be reduced by using short length wire with suture system and by minimizing time interval between procedure to operation. Alternatively, localization with radiocontrast materials, such as lipiodol, might be an option for ensuring accurate resection margins using intraoperative cone-beam CT or C-arm fluoroscopy while stapling. However, this method has also several disadvantages, including a potential risk of dye embolism, and is only available in hybrid operating rooms (cone beam CT or C-arm fluoroscopy) (26,27). To compensate for the disadvantages of solo localization with only hook-wires or lipiodol, we planned a clinical study involving a dual localization technique with both hook-wires and lipiodol use, guided via CT fluoroscopy for VATS resection of small pulmonary nodules. Our method was performed in 32 patients with 36 nodules. Under CT-fluoroscopy guidance, 0.2 mL of lipiodol was injected into the center of the lesion, followed by hook-wire (21-gauge needle, Kopans Breast Lesion Localization needle; Cook Medical, Bloomington, IN, USA) insertion at the same site (Figure 1). There was no catastrophic event such as air embolism following hookwire insertion in our study.
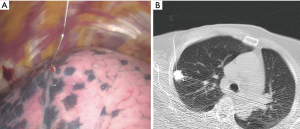
During surgical resection, we identified the pleural location via the hook-wire and ensured adequate resection margins using lipiodol spots under intraoperative C-arm fluoroscopy. Using this method, we reported that all lesions were successfully resected; however, one case involved hook-wire dislodgement and could be identified by observing the lipiodol under intraoperative C-arm fluoroscopy. The mean resection margin was 4.9 mm and additional resection was performed in three cases for further clearance of resection margins. Lipiodol marking could provide surgeons clearer target spot images so as to achieve more adequate surgical resection margins during therapeutic sublobar resection (28). Wire dislodgement or migration
Dual localization techniques using radiotracers and hook wires
We reported the feasibility of VATS resection for small subpleural pulmonary nodules after dual localization using CT-fluoroscopy-guided hook-wire insertion and radiotracer use; (99m)Tc-phytate. Usually, the hook-wire marking technique has been showed acceptable success rates of target localization in several studies; however, there have been problems related with hook-wire insertion, such as displacement and pulmonary hemorrhage, which are critical for VATS resection. Thus, we performed the dual localization technique to compensate for the limitation of hook wire localization using the dual localization method with additional radiotracer markers on 36 pulmonary nodules in 34 patients (19). In our trial, hook-wire marking was used for the detection of pleural location. Subsequently, after the removal of the hook-wire, we investigated radioactivity with intraoperative hand-held gamma probes (Neo2000; Devicor, Cincinnati, OH, USA). After resection, we studied the yield using this method, with the pathologic results. Hook-wire dislodgement occurred in seven cases; however, we resected the target lesion successfully by gamma probe-guided identification in all seven cases. This technique uses radiotracers as a marking material, which need hand-held gamma probes. The use of radiocontrast materials, such as lipiodol, usually need a hybrid operation setting for intraoperative detection (cone beam CT or C-arm fluoroscopy; huge devices), which is not easily available. The potential disadvantage of both techniques described here is radiation exposure to patients from the radiotracers, intraoperative CT, or C-arm fluoroscopy (18,29).
Intraoperative tumor detection by systemic injection of fluorescent dye
Recently, near infrared fluorescence (NIR) imaging has become an emerging technique for the localization for lung lesion. Originally, fluorescent dyes, such as indocyanine green (ICG), have been used for medical diagnosis to determine cardiac output (30), hepatic function (31), or during ophthalmic angiography (32). ICG has a peak spectral absorption at about a wavelength of 800 nm (near infrared). ICG has been used for the visualization of tumor extent and cancer cells in cancer surgery (33,34). The visualization of the extent to which lymph nodes harbor tumor cells is frequently performed in breast cancer (35) or skin tumor (36) using ICG injection to determine the surgical extent required. Several studies demonstrated the intraoperative visualization of NIR imaging a few hours or days after systemic injection of ICG in colorectal hepatic metastasis (37) and brain tumors (38).
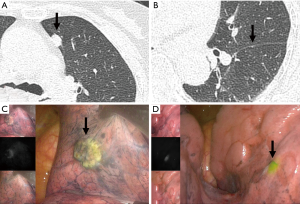
For NIR visualization of lung lesion, Okusanya and colleagues already demonstrated that pulmonary nodules might be visualized via intraoperative NIR imaging by the systemic injection of ICG (5 mg/kg), 1 day before operation (39). However, their use of 5 mg/kg ICG is the maximum limit for humans and sometimes a high dose might be associated with toxicity or allergic reaction to iodine. In our preclinical trials, we demonstrated that the perfusion of vital organs could be imaged using 0.6 mg/kg ICG via NIR imaging in a porcine model. From the early preclinical experiences, we studied the optimal human-dose of ICG for intraoperative NIR imaging by reducing it to 1 mg/kg ICG, 1 day before surgery. In 11 patients, we evaluated intraoperative NIR imaging and measured fluorescence intensities of lung lesions using endoscopic fluorescence imaging systems. From the study, we found that a low dose (1 mg/kg) of ICG was sufficient for intraoperative NIR imaging of pulmonary tumors (40). However, the fluorescent signal was not correlated with the tumor pathology, size, or depth from pleura. We could see the fluorescent signal on 3-mm sizes nodule which was the smallest in our series. However, our result did not discriminate the tumor from inflammatory lesions on intraoperative NIR images with ICG (Figure 2). The limitation of this study was the nonspecific NIR signal of inflammatory lesion. Future study will focus on how we could incorporate the intravenous ICG technique in real clinical field for detection of pulmonary lesion.
Visualization of intersegmental planes during segmentectomy with fluorescent dye
To define the intersegmental plane during the segmentectomy, NIR fluorescence imaging techniques with ICG could provide a clear real-time imaging for intraoperative use. The various techniques for intersegmental plane visualization during segmentectomy have already been described in several studies (41,42). For minimally invasive thoracic surgery, dye-based methods using fluorescent dyes, such as ICG, could provide clear real-time NIR images. Several studies reported that the use of ICG injection for NIR imaging of intersegmental planes for VATS segmentectomy (43-45). We studied real-time intraoperative detection of intersegmental planes with endoscopic NIR images by the systemic injection of ICG after pulmonary artery division of target segments (Figure 3).
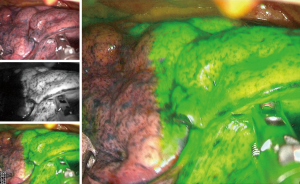
The optimal dose of ICG for intersegmental plane visualization in the clinical setting was evaluated in animal models, and we also found that an ICG concentration of 0.6 mg/kg was sufficient for fluorescent imaging (46).
Intraoperative localization under CT guidance with fluorescent dye and lipiodol
There were some attempts to combine dye and contrast agent without hook wire (47,48), the fluorescent images have been reported to be used for preoperative localization of lung nodules. However, soluble ICG can spread out through the lung parenchyma without staying the injected lesion. In addition, it is difficult to decide the resection margin of deep-seated nodule for limited depth penetration of fluorescent images (13,38).
Thus, to overcome these limitations, we developed fluorescent iodized emulsion by mixing ICG dye with lipiodol (lipid soluble radio contrast) to remain ICG on the injected target and to reduce the depth limitation (Figure 4). In prospective trial of 36 nodules, ICG signal was not detected in two patients, but these two nodules were detected and successfully resected under C-arm fluoroscopic guidance.

Intraoperative localization via ENB with fluorescent dye and lipiodol
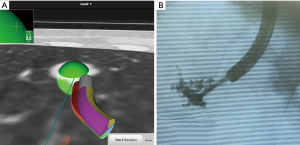
More recently, ENB has been used to help localize the target lesion or visualize the intersegmental plane during VATS-based surgical resection (Figure 5). Originally, ENB was developed as a minimally invasive diagnostic tool for peripheral lung lesion with less procedure-related complications. The use of ENB has been increasing for localization of target lung lesions during VATS or robotic sublobar resection (49-51). In recent series of ENB localization for VATS resection, they reported the acceptable results using methylene blue, indigo carmine or ICG. However, soluble dye injection by ENB technique might show the dye diffusion which could not see the correct target location. In our study, we studied the efficacy of mixed dye with ICG and lipiodol to prevent the dye diffusion during ENB localization. We performed ENB localization with ICG for VATS or robotic resection in 10 patients. The ICG and lipiodol was mixed with in the ratio of 1:9 and injected 1 mL of mixed dye to target lesion using 1.9-mm diameter injection needle. Using this technique, our preliminary data showed a 90% success rate and one failure, probably because the target lesion was located in the deep lung parenchyma (34-mm from the pleura) and there was no adverse event and no dye diffusion. Although we need further studies to ascertain the role of the ENB localization technique as a virtual image guiding tool for minimally invasive sublobar resection, this technique using fluorescent dyes and mixed dye to minimize diffusion, might be a promising tool for future image-guided thoracic surgery.
Summary
In this article, we described our preclinical and clinical applications of imaging techniques for thoracic surgery. Although our preclinical or clinical experiences might not demonstrate all innovations in image-guided techniques for thoracic surgery, current techniques for image-guided surgery might help thoracic surgeons perform minimal resections with minimal incision to improve surgical outcomes. Recent applications of ENB for localization will enable surgeon-led localization with high accuracy and patient’s safety; it will also facilitate patients-specific surgery in the near future.
Acknowledgements
Funding: This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI17C0654), and a National Research Foundation of Korea (NRF) grant funded by the Ministry of Education, Science and Technology (NRF-2015R1A2A2A04005760).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mezger U, Jendrewski C, Bartels M. Navigation in surgery. Langenbecks Arch Surg 2013;398:501-14. [Crossref] [PubMed]
- Galloway Jr RL. Chapter 1 - Introduction and Historical Perspectives on Image-Guided Surgery A2. In: Golby AJ. editor. Image-Guided Neurosurgery. Boston: Academic Press, 2015:1-22.
- Swanson SJ. Video-Assisted Thoracic Surgery Segmentectomy: The Future of Surgery for Lung Cancer? Ann Thorac Surg 2010;89:S2096-7. [Crossref] [PubMed]
- Rami-Porta R, Tsuboi M. Sublobar resection for lung cancer. Eur Respir J. 2009;33:426-35. [Crossref] [PubMed]
- Ampel NM. The solitary pulmonary nodule. N Engl J Med. 2003;349:1575-author reply 1575. [Crossref] [PubMed]
- Yang W, Jiang H, Khan AN, et al. Transthoracic needle aspiration in solitary pulmonary nodule. Transl Lung Cancer Res 2017;6:76-85. [Crossref] [PubMed]
- Rice TW. The solitary pulmonary nodule: accurate diagnosis allows appropriate treatment. Semin Thorac Cardiovasc Surg 2002;14:238. [Crossref] [PubMed]
- Skouras VS, Tanner NT, Silvestri GA. Diagnostic approach to the solitary pulmonary nodule. Semin Respir Crit Care Med 2013;34:762-9. [Crossref] [PubMed]
- Swanson SJ, Jaklitsch MT, Mentzer SJ, et al. Management of the solitary pulmonary nodule: role of thoracoscopy in diagnosis and therapy. Chest 1999;116:523S-4S. [Crossref] [PubMed]
- Cardillo G, Regal M, Sera F, et al. Videothoracoscopic management of the solitary pulmonary nodule: a single-institution study on 429 cases. Ann Thorac Surg 2003;75:1607-11; discussion 1611-2. [Crossref] [PubMed]
- Mattioli S, D'Ovidio F, Daddi N, et al. Transthoracic endosonography for the intraoperative localization of lung nodules. Ann Thorac Surg 2005;79:443-9; discussion 443-9. [Crossref] [PubMed]
- Wada H, Anayama T, Hirohashi K, et al. Thoracoscopic ultrasonography for localization of subcentimetre lung nodules. Eur J Cardiothorac Surg 2016;49:690-7. [Crossref] [PubMed]
- Zhou J, Yang F, Jiang GC, et al. Applications of indocyanine green based near-infrared fluorescence imaging in thoracic surgery. J Thorac Dis 2016;8:S738-43. [Crossref] [PubMed]
- Chiu CH, Chao YK, Liu YH, et al. Clinical use of near-infrared fluorescence imaging with indocyanine green in thoracic surgery: a literature review. J Thorac Dis 2016;8:S744-8. [Crossref] [PubMed]
- Kidane B, Yasufuku K. Advances in Image-Guided Thoracic Surgery. Thorac Surg Clin 2016;26:129-38. [Crossref] [PubMed]
- Lin MW, Tseng YH, Lee YF, et al. Computed tomography-guided patent blue vital dye localization of pulmonary nodules in uniportal thoracoscopy. J Thorac Cardiovasc Surg 2016;152:535-44.e2. [Crossref] [PubMed]
- Lenglinger FX, Schwarz CD, Artmann W. Localization of pulmonary nodules before thoracoscopic surgery: value of percutaneous staining with methylene blue. AJR Am J Roentgenol 1994;163:297-300. [Crossref] [PubMed]
- Bellomi M, Veronesi G, Trifiro G, et al. Computed tomography-guided preoperative radiotracer localization of nonpalpable lung nodules. Ann Thorac Surg 2010;90:1759-64. [Crossref] [PubMed]
- Doo KW, Yong HS, Kim HK, et al. Needlescopic resection of small and superficial pulmonary nodule after computed tomographic fluoroscopy-guided dual localization with radiotracer and hookwire. Ann Surg Oncol 2015;22:331-7. [Crossref] [PubMed]
- Ciriaco P, Negri G, Puglisi A, et al. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg 2004;25:429-33. [Crossref] [PubMed]
- Powell TI, Jangra D, Clifton JC, et al. Peripheral lung nodules: fluoroscopically guided video-assisted thoracoscopic resection after computed tomography-guided localization using platinum microcoils. Ann Surg 2004;240:481-8; discussion 488-9. [Crossref] [PubMed]
- Ng CSH, Chu CM, Lo CK, et al. Hybrid operating room Dyna-computed tomography combined image-guided electromagnetic navigation bronchoscopy dye marking and hookwire localization video-assisted thoracic surgery metastasectomy. Interact Cardiovasc Thorac Surg 2018;26:338-40. [Crossref] [PubMed]
- Sun J, Mao X, Xie F, et al. Electromagnetic navigation bronchoscopy guided injection of methylene blue combined with hookwire for preoperative localization of small pulmonary lesions in thoracoscopic surgery. J Thorac Dis 2015;7:E652-6. [PubMed]
- Chen S, Zhou J, Zhang J, et al. Video-assisted thoracoscopic solitary pulmonary nodule resection after CT-guided hookwire localization: 43 cases report and literature review. Surg Endosc 2011;25:1723-9. [Crossref] [PubMed]
- Horan TA, Pinheiro PM, Araújo LM, et al. Massive gas embolism during pulmonary nodule hook wire localization. Ann Thorac Surg 2002;73:1647-9. [Crossref] [PubMed]
- Yang SM, Ko WC, Lin MW, et al. Image-guided thoracoscopic surgery with dye localization in a hybrid operating room. J Thorac Dis 2016;8:S681-9. [Crossref] [PubMed]
- Stanzi A, Mazza F, Lucio F, et al. Tailored intraoperative localization of non-palpable pulmonary lesions for thoracoscopic wedge resection using hybrid room technology. Clin Respir J 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Kang D-Y, Kim HK, Kim YK, et al. Needlescopy-assisted resection of pulmonary nodule after dual localisation. Eur Respir J 2011;37:13-7. [Crossref] [PubMed]
- Grogan EL, Jones DR, Kozower BD, et al. Identification of small lung nodules: technique of radiotracer-guided thoracoscopic biopsy. Ann Thorac Surg 2008;85:S772-7. [Crossref] [PubMed]
- Maarek JM, Holschneider DP, Harimoto J, et al. Measurement of cardiac output with indocyanine green transcutaneous fluorescence dilution technique. Anesthesiology 2004;100:1476-83. [Crossref] [PubMed]
- De Gasperi A, Mazza E, Prosperi M. Indocyanine green kinetics to assess liver function: Ready for a clinical dynamic assessment in major liver surgery? World J Hepatol 2016;8:355-67. [Crossref] [PubMed]
- Ciardella AP, Prall FR, Borodoker N, et al. Imaging techniques for posterior uveitis. Curr Opin Ophthalmol 2004;15:519-30. [Crossref] [PubMed]
- Marshall MV, Rasmussen JC, Tan IC, et al. Near-Infrared Fluorescence Imaging in Humans with Indocyanine Green: A Review and Update. Open Surg Oncol J 2010;2:12-25. [Crossref] [PubMed]
- Nguyen QT, Tsien RY. Fluorescence-guided surgery with live molecular navigation — a new cutting edge. Nat Rev Cancer 2013;13:653. [Crossref] [PubMed]
- Hirche C, Murawa D, Mohr Z, et al. ICG fluorescence-guided sentinel node biopsy for axillary nodal staging in breast cancer. Breast Cancer Res Treat 2010;121:373-8. [Crossref] [PubMed]
- Urbanska K, Romanowska-Dixon B, Matuszak Z, et al. Indocyanine green as a prospective sensitizer for photodynamic therapy of melanomas. Acta Biochim Pol 2002;49:387-91. [PubMed]
- van der Vorst JR, Schaafsma BE, Hutteman M, et al. Near-infrared fluorescence-guided resection of colorectal liver metastases. Cancer 2013;119:3411-8. [Crossref] [PubMed]
- Vahrmeijer AL, Hutteman M, van der Vorst JR, et al. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol 2013;10:507. [Crossref] [PubMed]
- Okusanya OT, Holt D, Heitjan D, et al. Intraoperative near-infrared imaging can identify pulmonary nodules. Ann Thorac Surg 2014;98:1223-30. [Crossref] [PubMed]
- Kim HK, Quan YH, Choi BH, et al. Intraoperative pulmonary neoplasm identification using near-infrared fluorescence imaging. Eur J Cardiothorac Surg 2016;49:1497-502. [Crossref] [PubMed]
- Zhang Z, Liao Y, Ai B, et al. Methylene Blue Staining: A New Technique for Identifying Intersegmental Planes in Anatomic Segmentectomy. Ann Thorac Surg 2015;99:238-42. [Crossref] [PubMed]
- Sekine Y, Ko E, Oishi H, et al. A simple and effective technique for identification of intersegmental planes by infrared thoracoscopy after transbronchial injection of indocyanine green. J Thorac Cardiovasc Surg 2012;143:1330-5. [Crossref] [PubMed]
- Misaki N, Chang SS, Igai H, et al. New clinically applicable method for visualizing adjacent lung segments using an infrared thoracoscopy system. J Thorac Cardiovasc Surg 2010;140:752-6. [Crossref] [PubMed]
- Tarumi S, Misaki N, Kasai Y, et al. Clinical trial of video-assisted thoracoscopic segmentectomy using infrared thoracoscopy with indocyanine green. Eur J Cardiothorac Surg 2014;46:112-5. [Crossref] [PubMed]
- Pardolesi A, Veronesi G, Solli P, et al. Use of indocyanine green to facilitate intersegmental plane identification during robotic anatomic segmentectomy. J Thorac Cardiovasc Surg 2014;148:737-8. [Crossref] [PubMed]
- Oh Y, Quan YH, Kim M, et al. Intraoperative fluorescence image-guided pulmonary segmentectomy. J Surg Res 2015;199:287-93. [Crossref] [PubMed]
- Jin KN, Lee KW, Kim TJ, et al. Computed tomography guided percutaneous injection of a mixture of lipiodol and methylene blue in rabbit lungs: evaluation of localization ability for video-assisted thoracoscopic surgery. J Korean Med Sci 2014;29:129-36. [Crossref] [PubMed]
- Klijian AS. Agar blue localization of small pulmonary nodules and ground glass opacifications for thoracoscopic resection. J Thorac Dis 2016;8:S677-80. [Crossref] [PubMed]
- Andrade RS. Electromagnetic navigation bronchoscopy-guided thoracoscopic wedge resection of small pulmonary nodules. Semin Thorac Cardiovasc Surg 2010;22:262-5. [Crossref] [PubMed]
- Muñoz-Largacha JA, Ebright MI, Litle VR, et al. Electromagnetic navigational bronchoscopy with dye marking for identification of small peripheral lung nodules during minimally invasive surgical resection. J Thorac Dis 2017;9:802-8. [Crossref] [PubMed]
- Marino KA, Sullivan JL, Weksler B. Electromagnetic Navigation Bronchoscopy for Identifying Lung Nodules for Thoracoscopic Resection. Ann Thorac Surg 2016;102:454-7. [Crossref] [PubMed]


