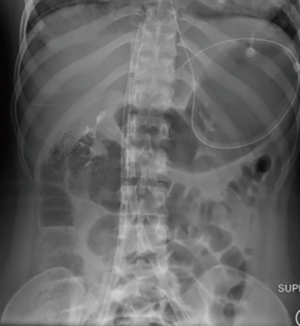
Cannulation technique: femoro-femoral
Introduction
Optimal cannulation technique is essential for the initiation and management of patients with severe respiratory failure requiring veno-venous extracorporeal membrane oxygenation (VV ECMO). Cannulation must be rapid and with a technique that minimizes tissue trauma, which can lead to bleeding and transfusion requirements, reoperation, infection risk and longer-term morbidity. Inappropriately positioned cannulas can result in poor circuit flows, recirculation and inadequate support. Moving malpositioned cannulas can expose the patient to non-sterile parts of the cannula and increase the risk of infection. Poorly secured cannulas can lead to catastrophic decannulation. In the following discussion, we will describe in detail the VV ECMO cannulation techniques that have been developed in our hospital in over 25 years of practice and discuss the various advantages and disadvantages of each technique.
Surgical versus percutaneous cannulation
The surgical or “open” cut down procedure was the preferred method of venous cannulation until the 1990s (1). This technique could either be a direct cut down to expose the vessel, which required a large incision, or a smaller proximal incision to expose the vessels, with a second more distal incision to enable percutaneous tunneling of the cannulas to the vessel opening. This facilitated a lower angle of incidence with the vessel, and reduced infection risk and bleeding (2). The advantages of the surgical technique include direct visualization of the anatomy, confirmation of cannula entry into the vessels, and purse string sutures for haemostasis (3).
Since the 1990s, the development of thin-walled wire-reinforced cannulas and improved quality and availability of ultrasound (US) machines has enabled the percutaneous technique to become more widespread. Rather than moving an unstable patient to the operating room, clinicians can perform the cannulation at the patient’s bedside, which may be in the intensive care unit (ICU) or emergency department (4). More importantly, there is an awareness that the amount of bleeding, infection risk, lymphocele and timeliness can be improved (see Table 1)

Full table
Femoro-femoral VV ECMO cannulation
The femoro-femoral (“fem-fem” or bifemoral) VV ECMO cannulation configuration has both the access (drainage) and return cannula inserted via the common femoral veins. Blood is accessed from one cannula positioned in the inferior venous cava (IVC), pumped through the oxygenator of the ECMO circuit and returned to the patient at the level of the right atrium (RA) via the contralateral femoral vein.
Advantages of fem-fem configuration
There are a number of factors that make the fem-fem configuration advantageous for VV ECMO (Table 2).
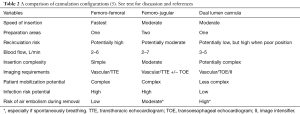
Full table
Speed and simplicity of circuit
The left and right femoral veins are usually large and easily accessible and enable rapid central access for the initiation of VV ECMO. Both veins are accessible at the groin so only one area of the body requires preparation. Furthermore, the femoral area allows rapid access to the femoral artery if veno-arterial (VA) ECMO support is required. Fem-fem cannulation can also be inserted without the requirement of transoesophageal echocardiogram (TOE) or radiology, which add to the complexity of cannulation and may be difficult to arrange during ECMO retrievals.
Safety and complications
All ECMO cannulation is associated with significant risks to the patient. The advantage of the femoral veins is that they are almost always accessible and require less skill for insertion. Adverse effects such as bleeding can usually be controlled with local pressure. The main alternative to femoral cannulation includes the internal jugular vein (IJV), which is used in both femoro-jugular (fem-jug) and dual lumen configurations. The major risk is pneumothorax, which although uncommon, can be fatal in patients with severe respiratory failure. The large diameter dual lumen catheter can be particularly challenging to place. Accidental distal hepatic vein or right ventricle cannulation can occur from misplaced wires and dilators, and can result in catastrophic bleeding or pericardial tamponade, as occurred in two patients out of 72 cannulations in one study (2). Other specific complications to IJV cannulation include cannula movement, cerebral venous congestion and air embolism upon removal (6). Patients with long-term central venous lines may also develop superior vena cava (SVC) stenosis which poses significant risk during IJV cannulation but is often not easy to exclude prior to cannulation.
ECMO blood flow
The maximum blood flow of an ECMO circuit is particularly important in patients with high cardiac outputs and severe respiratory failure: if the circuit cannot capture a sufficient proportion of cardiac output, then ongoing hypoxia is likely (7). The fem-fem configuration can support between 2–6 L/min of blood flow which is usually adequate. The fem-jug configuration may theoretically enable higher blood flows, as the return cannula directs flow directly across the tricuspid valve, and was found to have higher flows than atrio-femoral configurations (8). However, in a recent retrospective study, blood flows were similar (4.1 vs. 4.0 L/min, P=0.5) between fem-fem and fem-jug configurations (9). Blood flow rates are usually limited by flow into the access cannula which likely explains this non-significant difference between the two configurations. The dual lumen catheter also has more limited maximum flows [typically, the 23 Fr cannula allows a maximum flow of 3 L/min, the 27 Fr cannula is limited to 4.5 L/min, and the 31 Fr double lumen bicaval (DLB) cannula allows not more than 5 L/min (5)]. In practice, a poorly positioned dual lumen cannula can result in low ECMO flows and/or high recirculation, resulting in hypoxia. In our practice, if inadequate flow is results in hypoxia following a fem-fem cannulation, we insert an additional IJV access cannula. This “high flow” configuration enables improved blood flow and oxygenation (10).
Interhospital and intrahospital transport
The transport of ECMO patients is logistically complex and associated with multiple risks for the patient (11). This may occur in interhospital transports, or in intrahospital transports to the operating room (OR) or to radiology. The fem-fem configuration allows for positioning of the ECMO console at the foot of the bed, either attached to the bed itself or on a stretcher bridge. All tubing can be safely positioned on the lower half of the bed away from the patient’s head and airway. In both dual lumen and fem-jug configurations there is a requirement for tubing to run near to the patient’s head along with ventilation circuits. In the fem-jug configuration tubing needs to run along the length of the patient whether the console is positioned at the head or the foot of the bed. This can lead to difficulties turning the patient, accidental cannula dislodgment, or kinking of tubing with circuit compromise.
Disadvantages of fem-fem configuration (Figure 1)
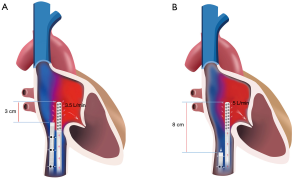
Recirculation
Recirculation occurs when returned blood is withdrawn though the access cannula rather than flowing forward through the pulmonary circulation (12). In fem-fem cannulation, the return blood is directed toward the SVC rather than the tricuspid valve, potentially creating abnormal flows away from the valve and more recirculation. Fem-jug and dual lumen configurations have potentially less recirculation as they direct the blood towards the tricuspid valve. In practice, we have found that recirculation is rarely a problem if ≥8 cm of separation of cannulas tips in the inferior vena cava (IVC) can be maintained.
Infection
Although there is no definitive comparative data on risk of infection, extrapolating rates from central venous catheters (CVCs) may suggest that infection rates may be higher with dual femoral access (13) compared with single access in the IJV. The dual lumen cannula may therefore have the lowest rate of infection when compared to configurations that require femoral cannulation.
Challenging access and cannula position
Morbidly obese patients can make exposure of the femoral vessels challenging, and IVC filters are a contraindication to femoral cannulation, and require alternative access. Taller patients may have a distance from groin to the RA/IVC junction that exceeds the length of a cannula.
Mobilization
Femoral cannulation was traditionally thought to be a contraindication for mobilization. However, we have found that this can be done safely if appropriately trained staff are present. A key advantage of the dual lumen cannula is that mobilization can be more easily managed.
Cannula selection
Cannula selection is a critical component of any ECMO configuration. Fem-fem ECMO requires the selection of two long (50–55 cm) cannula capable of reaching the central circulation. Consideration needs to be given to the desired ECMO blood flow, size of the vessels, as well as the percentage of recirculation.
Options for the access limb include both multi-stage (multiple access points) and single-stage (access only from tip of catheter) cannula. Only a single stage cannula should be used for the return limb of the circuit, as multistage return cannulas cause significant recirculation. The multistage cannula has a flow profile that allows blood to enter via a number of holes along the distal length of the cannula (usually 20–30 cm). In the femoral position, this means that blood can be accessed not only from the distal tip of the cannula in the IVC, but all the way down to the iliac vessels. The theoretical advantage of this catheter is that it is less likely to be compromised by access insufficiency, therefore maintaining high access flows (14). It also has the ability to access blood a distance from the central circulation, resulting in less recirculation.
Sizing
Increasing cannula diameter will improve the maximum blood flow through the circuit but is also more likely to result in vessel and tissue damage. The size of the cannula on the access side of the circuit is especially important, as cannula that are too small result in impaired blood flow, higher revolutions per minute (RPM) with potential for blood trauma, and access insufficiency. Several methods of estimating cannula size can be used. One method is to calculate the patients cardiac output, and then estimate the peak flow through a cannula using the information provided by the manufactures. Our technique is to place the largest cannulas possible that are safe, based on the size of the femoral vessels. We do this by measuring the diameter of the femoral vessels on US. The diameter of the femoral vessel at the insertion point is measured and the circumference of the vessel calculated using the formula πD. The result in millimeters will give the largest French size cannula capable of being passed. For example:
- Measured vessel diameter =10 mm;
- Calculated circumference: πD =3.1×10 mm =31 mm;
- Max size of cannula =31 F.
It is important that the cannula is smaller than the maximum size of the vessel in order to allow some venous drainage around the cannula (3). If ≥2/3 of the vessel is occluded by the cannula, then oedema, stasis, and severe venous hypertension can lead to deep vein thrombosis, lower limb venous congestion and catastrophic ischemic injury. For the majority of patients, we use 19–25 Fr access and 17–21 Fr return.
Site selection
There is no clear evidence that one side is better than the other when choosing access or return orientation. Factors to consider are that the left femoral/iliac vein is more tortuous than the right, the angle with the IVC is sharper, and it is marginally longer as it crosses in front of the aorta (see Figure 2). The length of the vein in particular can lead to problems during advancing and positioning of the cannulas. In general, we place the access cannulas on the left in small stature patients as the shorter right side may lead to exposure of the proximal multistage holes and air embolism. And return cannulas are in general placed on the right in tall stature patients as a left sided return cannula may not reach the RA/IVC junction, leading to reduced separation (
The insertion technique
We prefer an US-guided percutaneous insertion technique, with serial dilation using a Seldinger technique (15). There is no skin incision. This technique results in a snug passage of the cannula through both the skin and femoral sheath. This technique results in minimal bleeding and has the theoretical advantage of decreased risk of infection, particularly for a long VV ECMO run. Using this technique, we have found near 100% successful cannulation rates. This has been replicated in other groups (16).
US
US-guided vascular access has been shown to improve success rates for insertion of central venous lines, and decrease complications such as infections, mechanical complications, and thrombosis, compared to the landmark techniques (17). Avoiding these complications is even more important for ECMO cannulation, where large dilators and cannulas can cause significant damage and morbidity. While inadvertent arterial puncture is not usually a problem during CVC insertion, in unwell anticoagulated ECMO patients this can result in prolonged bleeding or hematoma formation requiring operative management (18).
US and insertion technique
Two trained cannulators perform the procedure. Both groins are prepared, cleaned and draped.
We employ both vascular US with a linear probe for the guided insertion of wires and transthoracic echocardiography with a phased array probe to confirm the wires are in the correct vessel and to position the cannulas (19). We begin with a pre-scan (an US of the vessels prior to sterile scrubbing) to assess for any barriers to cannulation, such as small size of the vessels, thrombosis or stenosis. US is then used to guide the shallow angle passage of the needle into the common femoral vein in real-time, by continuously visualizing the tip of the needle in the US field (20). This technique ensures a single pass of the needle into the vessel. Non-visualised techniques of insertion into the vessel may result in steep or off-center penetration that can make subsequent dilation more difficult and increases the risk of vessel perforation or kinking of the guide wire.
We use soft 140–180 cm J-tipped wires to minimize the risk of damage to venous structures or the heart and confirm their position in the IVC using a subcostal view of the heart. We also intermittently check the position of the wires throughout the procedure to confirm there has been no wire migration. In difficult cases, such as tortuous vessel or repeated kinking of the wire, we may employ stiffer wires (such as an Amplatz Super StiffTM Guidewire, Boston Scientific, USA). However, this requires careful monitoring to ensure the wires do not migrate inwards as the risks of internal damage are higher with a stiffer, straight tipped wire.
Serial dilation (Figure 3)
Without the use of a skin incision, we serially dilate the cannula passage, stepping up by one dilator (2 French sizes) sequentially leading to a gradual stretching of the skin, subcutaneous tissue and insertion point into the vessel. The primary cannulator advances the dilator, while the second cannulator continuously moves the wire in and out of the dilator, ensuring the wire is not being kinked. If kinking begins to occur, there is increased resistance of the wire being moved in and out. The second cannulator can inform the primary cannulator to retract the dilator and adjust the technique. If a kink forms on the wire, the wire should be withdrawn a few centimeters past the kink prior to continuation of cannulation (14).
At the end of cannula insertion, we use US to assess for any complications which may have occurred during the procedure, such as hematoma formation, pseudoaneurysm, or arterial transection, and therefore steps can be taken to mitigate these effects.
Cannula positioning (Figure 4)
Correct cannula positioning is essential for effective VV ECMO blood flow. It is important to get this correct the first time, as advancing the cannulas later can result in loss of sterile field and infection risk. In all forms of VV ECMO the return cannula needs to be positioned in the RA. This allows for oxygenated returning blood from the extra-corporeal oxygenator to be directed into the right heart and subsequently pumped into the pulmonary circulation.
Positioning of the access cannula in fem-fem cannulation can require some skill. The cannula tip needs to be positioned high enough into the central venous circulation to maximise blood flow, but far enough away from the return cannula to minimise recirculation. We aim to position the tip of the access cannula in the IVC just distal to the junction of the hepatic vein. At this site, the IVC is often non-collapsible even when hypovolemic as it is held open by the liver architecture. In this position there should be 7–10 cm of separation between the tips of the two cannulas (with the tip of the return cannula 4–5 cm into the RA).
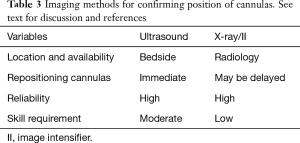
Imaging for positioning
There are a number of modalities that can be used to confirm the position of cannula in the fem-fem configuration (see Table 3). These include both transthoracic echocardiogram (TTE) and transoesophageal echocardiogram (TOE) echocardiography, plain radiography and image intensifiers (II).
Full table
Underwater seal connections and administration of heparin
Once inserted, each cannula is flushed and locked with approximately 1,500 units of heparin (in 150 mL of normal saline) to prevent clot formation (total approx. 3,000 units). The heparin bolus is omitted in patients with active bleeding, and heparin is started once bleeding has settled. Next the cannulas are connected to the ECMO circuit using an “underwater seal” technique. This involves continuously dripping water from a syringe onto the connections as they are joined and ensures no air bubbles are present.
Cannula securing
Once the cannulas have been correctly positioned they are secured. Needle and suture techniques are commonly described, but can cause ischemia of the skin, are a nidus for infection and also can lead to inadvertent puncturing of the cannulas and/or circuit. We prefer to use a large Tegaderm at the skin insertion sites and self-adhesive dressings or “Grip-Locks”, in a minimum of two places securing the length of the cannula. It is important that the first Grip-Lock is not too far away from the insertion site as this can allow movement and the potential for inadvertent decannulation (21).
Sedation
All patients who undergo cannulation for VV ECMO should be intubated prior to the procedure. Patients requiring VV ECMO usually have a high work of breathing and the risk of air embolism during cannulation is high. Once cannulation is complete sedation may not be required. The patient may be able to be desedated and even extubated in some circumstances. As an example, in patients with cystic fibrosis, early extubation should be attempted to facilitate coughing and respiratory physiotherapy.
Explantation
Following a successful VV ECMO weaning study, the cannulas are removed using a manual compression technique. Heparin is stopped for 2 hours prior to removal (22). The patient should be positioned supine and prepared for the procedure. Care is taken to remove any clot that has formed on the tip of the cannula. Firm manual pressure for 20 minutes is used and sufficient in the majority of cases. If there is ongoing bleeding after this, then this is continued for a further 20 minutes. Rarely a suture can be placed, or operative management is required for recalcitrant bleeding (23).
Training and accreditation
VV ECMO cannulation is a technically challenging and highly invasive procedure with high inherent risks for bleeding, tissue injury and air embolism. All staff undergo regular training, accreditation, supervision, and practice including in the animal lab to ensure the procedure is done in a standardised manner to minimize the potential risk to patients.
Conclusions
US-guided percutaneous fem-fem cannulation has advantages of being quick and simple to insert, with minimal complications and adequate blood flows in the majority of patients. Disadvantages include insufficient blood flow in a subset of patients, and the potential for recirculation. VV ECMO cannulation requires a balanced analysis of these factors in order to tailor the correct techniques for the right patient.
Acknowledgements
The authors wish to thanks Dr. Tim Byrne for his help with Figure 1. We would also like to give our sincere thanks to all nurses, physiotherapists and physicians for their outstanding commitment to the care of our critically ill patients.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Migliari M, Marcolin R, Avalli L, et al. Percutaneous Cannulation: Indication, Technique, and Complications. ECMO extracorporeal life support in adults. Springer-Verlag Italia, 2014.
- Reeb J, Olland A, Renaud S, et al. Vascular access for extracorporeal life support: tips and tricks. J Thorac Dis 2016;8:S353-63. [Crossref] [PubMed]
- Stulak JM, Dearani JA, Burkhart HM, et al. ECMO cannulation controversies and complications. Semin Cardiothorac Vasc Anesth 2009;13:176-82. [Crossref] [PubMed]
- Burrell AJ, Pellegrino VA, Sheldrake J, et al. Percutaneous Cannulation in Predominantly Venoarterial Extracorporeal Membrane Oxygenation by Intensivists. Crit Care Med 2015;43:e595. [Crossref] [PubMed]
- Banfi C, Pozzi M, Siegenthaler N, et al. Veno-venous extracorporeal membrane oxygenation: cannulation techniques. J Thorac Dis 2016;8:3762-73. [Crossref] [PubMed]
- Tulman DB, Stawicki SP, Whitson BA, et al. Veno-venous ECMO: a synopsis of nine key potential challenges, considerations, and controversies. BMC Anesthesiol 2014;14:65. [Crossref] [PubMed]
- Schmidt M, Tachon G, Devilliers C, et al. Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intensive Care Med 2013;39:838-46. [Crossref] [PubMed]
- Rich PB, Awad SS, Crotti S, et al. A prospective comparison of atrio-femoral and femoro-atrial flow in adult venovenous extracorporeal life support. J Thorac Cardiovasc Surg 1998;116:628-32. [Crossref] [PubMed]
- Guervilly C, Dizier S, Thomas G, et al. Comparison of femorofemoral and femorojugular configurations during venovenous extracorporeal membrane oxygenation for severe ARDS. Intensive Care Med 2014;40:1598-9. [Crossref] [PubMed]
- Ichiba S, Peek GJ, Sosnowski AW, et al. Modifying a venovenous extracorporeal membrane oxygenation circuit to reduce recirculation. Ann Thorac Surg 2000;69:298-9. [Crossref] [PubMed]
- Broman LM, Holzgraefe B, Palmér K, et al. The Stockholm experience: interhospital transports on extracorporeal membrane oxygenation. Crit Care 2015;19:278. [Crossref] [PubMed]
- Abrams D, Bacchetta M, Brodie D. Recirculation in venovenous extracorporeal membrane oxygenation. ASAIO J 2015;61:115-21. [Crossref] [PubMed]
- Parienti JJ, Mongardon N, Mégarbane B, et al. Intravascular Complications of Central Venous Catheterization by Insertion Site. N Engl J Med 2015;373:1220-9. [Crossref] [PubMed]
- Sidebotham D, Allen SJ, McGeorge A, et al. Venovenous extracorporeal membrane oxygenation in adults: practical aspects of circuits, cannulae, and procedures. J Cardiothorac Vasc Anesth 2012;26:893-909. [Crossref] [PubMed]
- Pellegrino V. Alfred ECMO Guideline. 2012:1-64. Available online: http://www.alfredicu.org.au/assets/Documents/ICU-Guidelines/ECMO/ECMOGuideline.pdf
- Conrad SA, Grier LR, Scott LK, et al. Percutaneous cannulation for extracorporeal membrane oxygenation by intensivists: a retrospective single-institution case series. Crit Care Med 2015;43:1010-5. [Crossref] [PubMed]
- Wu SY, Ling Q, Cao LH, et al. Real-time two-dimensional ultrasound guidance for central venous cannulation: a meta-analysis. Anesthesiology 2013;118:361-75. [Crossref] [PubMed]
- Bisdas T, Beutel G, Warnecke G, et al. Vascular complications in patients undergoing femoral cannulation for extracorporeal membrane oxygenation support. Ann Thorac Surg 2011;92:626-31. [Crossref] [PubMed]
- Platts DG, Sedgwick JF, Burstow DJ, et al. The role of echocardiography in the management of patients supported by extracorporeal membrane oxygenation. J Am Soc Echocardiogr 2012;25:131-41. [Crossref] [PubMed]
- Saugel B, Scheeren TWL, Teboul JL. Ultrasound-guided central venous catheter placement: a structured review and recommendations for clinical practice. Crit Care 2017;21:225. [Crossref] [PubMed]
- Rupprecht L, Lunz D, Philipp A, et al. Pitfalls in percutaneous ECMO cannulation. Heart Lung Vessel 2015;7:320-6. [PubMed]
- Yeo HJ, Kim HJ, Jang JH, et al. Vascular Complications Arising from Hemostasis with Manual Compression Following Extracorporeal Membrane Oxygenation Decannulation. J Card Surg 2016;31:123-6. [Crossref] [PubMed]
- Pracon R, Bangalore S, Henzel J, et al. A randomized comparison of modified subcutaneous "Z"-stitch versus manual compression to achieve hemostasis after large caliber femoral venous sheath removal. Catheter Cardiovasc Interv 2018;91:105-12. [Crossref] [PubMed]