Efficacy and safety of moxifloxacin in acute exacerbations of chronic bronchitis and COPD: a systematic review and meta-analysis
Introduction
Acute exacerbations of chronic bronchitis (AECB), including chronic obstructive pulmonary disease (AECOPD), represent a substantial health burden to patients, resulting in reduced lung function, increased morbidity and mortality, and long-term impairment in quality of life (1-3). Approximately around 40-50% of exacerbations may be attributed to bacteria while other causes include viral infections or environmental irritants (4). Current treatment guidelines recommend antibiotic therapy for patients with a more severe illness and often use acute symptom changes based on Anthonisen criteria of type I (worsening dyspnoea with increased sputum volume and purulence) or II (change in any two of these symptoms) exacerbations to define this group (5,6). The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendations for antibiotic therapy are based on the severity of exacerbations, the presence of risk factors, and predictors of poor outcome (e.g., comorbid conditions, frequency of AECBs, and previous antibiotic use) (7).
Moxifloxacin is a fourth-generation fluoroquinolone with a broad spectrum of activity against a wide range of the microorganisms isolated in AECB, including Gram-positive and Gram-negative bacteria, atypical pathogens, and anaerobic bacteria (8-10). Furthermore, moxifloxacin may be regarded as the most excellent tissue penetration ability (11). Several randomized controlled trials have been done to compare the effectiveness of moxifloxacin with various standard antimicrobials in the treatment of AECB (12-20). Most studies suggest that moxifloxacin has been approached as effective as standard antimicrobials (12-20). To date, few trials show clinical or bacteriological superiority of one antibiotic over another in AECB or AECOPD. Therefore, we performed a systematic literature review and meta-analysis to clarify whether the use of moxifloxacin could be associated with improved outcomes in comparison with standard antibiotic therapy in AECB or AECOPD.
Methods
Data sources and search strategy
To identify studies for inclusion in this review, two authors independently searched PubMed, the Cochrane Central Database of Controlled Trials, and EMBASE for relevant studies published up to July 2013. The search was limited to studies conducted with humans. No language restriction was imposed. Search terms were individualized for each database. Search terms used included: (“chronic bronchitis” OR “chronic obstructive pulmonary disease” OR “COPD”) AND (“moxifloxacin”). We also searched the proceedings of major relevant conferences, trial databases, the reference lists of identified trials, and major reviews.
Study selection
Two reviewers (K.X. Liu and B. Xu) independently screened studies for inclusion, retrieved potentially relevant studies, and determined study eligibility. Any discrepancies were resolved by consensus. Analysis was restricted to randomized controlled trials. For this meta-analysis, we considered those randomized control trials (RCTs) that compared the clinical efficacy of moxifloxacin and another antibiotic in patients with AECB and AECOPD. The definition of chronic bronchitis and COPD provided by each study was used. This entity was consistently defined as the presence of productive cough for at least three months in two consecutive years. While the definitions of an exacerbation were more varied, the patients consistently considered for inclusion in these studies were those who presented combinations of the key symptoms of exacerbation: increase in dyspnea, sputum volume, and sputum purulence with or without other minor symptoms. All of the studies considered patients with type I Anthonisen exacerbations for inclusion, and some also enrolled patients with type 2 or 3 exacerbations accompanied with increased in sputum purulence.
Data extraction
Two authors independently extracted data from all of the enrolled studies. Extracted data included study design (e.g., year conducted, sample size), patient characteristics, study methodology (e.g., eligibility criteria, method of randomization, and blinding), intervention (e.g., antimicrobial agents, dose, route of its administration and duration), and clinical outcomes. The primary outcome was clinical success (cure defined as resolution of all symptoms and signs of the bacterial exacerbation with a return to baseline condition, or improvement defined as subsidence of the ABECB but with an incomplete return to baseline condition) in intention-to-treat (ITT) and clinically evaluable (CE) patients. Treatment success was assessed at 6-21 days after initiation of antimicrobial treatment in order to avoid confounding due to spontaneous resolution of infection that occurs in half of the patients with AECB 21 days after the onset of infection. Treatment success in microbiologically evaluable patients (defined as the absence of pre-treatment isolated bacteria in sputum cultures) and pathogen eradication (documented or presumed) of the bacteria most frequently implicated in AECB isolates (namely Haemophilus influenzae, Moraxella catarrhalis and Streptococcus pneumoniae) were considered as secondary outcomes. When determining microbiological outcomes, we elected to assess them at the longest post-treatment time point reported in each eligible trial, in an attempt to capture possible relapses.
Quality assessment
We formally determined the methodological quality of each trial using the Jadad score (21), which incorporates randomization, blinding, and attrition to derive a score of 0 to 5; higher scores indicate higher quality. Two reviewers (K.X. Liu and B. Xu) independently appraised the quality of the included trials. Jadad score more than two was considered to denote good methodological quality of an RCT.
Statistical analysis
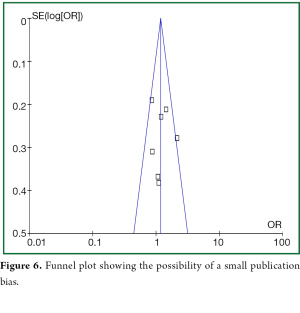
The meta-analysis was done using Review Manager 5.0 (Cochrane Collaboration, Oxford, UK). We computed pooled odds ratios (ORs) and 95% confidence intervals (CIs) from the adjusted ORs and 95% CIs reported in the observational studies. Potential heterogeneity should be achieved while we performing Cochrane Q and I2 statistics. We predefined heterogeneity as low, moderate, and high with I2 values of above 25%, 50%, and 75%, respectively. In the analysis of heterogeneity, we considered a P value <0.10 to be statistically significant. Study-level data were pooled using a random-effects model when I2 was >50% or a fixed-effects model when I2 was <50%. A funnel plot approaches of effect size vs. SE in the primary analysis of clinical success was employed to evaluate publication bias.
Results
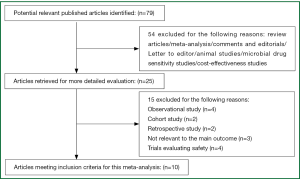
Our search obtained a total of 79 references. Of these potentially eligible studies, 10 met the criteria for inclusion in the meta-analysis (11-20). A flowchart for the studies evaluated and the reasons for exclusion are shown in Figure 1.
Study characteristics
The comparator antibiotics were amoxicillin/clavulanic acid, ceftriaxone, cefuroxime-axetil, clarithromycin and azithromycin (11-20). Five RCTs had double-blind (DB) designs (11,12,17-19), while five RCTs were open-labeled (13-16,20). Most of the studies included outpatients. Characteristics of the included studies are summarized in Table 1. All studies were published from 1999 to 2013. Trials were conducted in a diverse array of countries. The eligible trials enrolled patients experiencing an AECB classified as Anthonisen type I, II, III (11,12,14); or type I, II (13,15-17); or type I (18-20). In nine RCTs, data regarding the use of systemic corticosteroids before the occurrence of ABECB were provided. The average Jadad score of these studies was 3.5 (range: 1-5, Table 2).
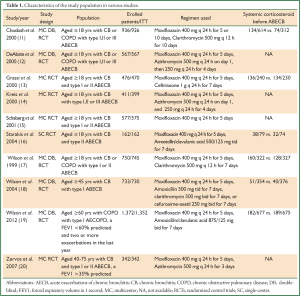
Full table
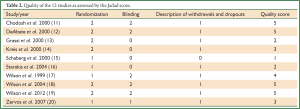
Full table
Outcomes of clinical and bacteriological success rates
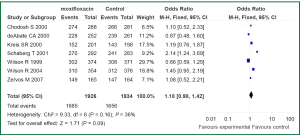
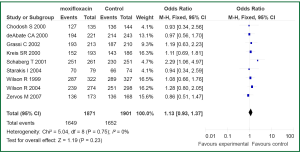
The primary outcome analysis was the clinical success rate at early follow-up in an ITT and CE populations. Early follow-up was before day 21 in all studies. Tests for statistical heterogeneity were performed for all analyses. Data regarding treatment success in ITT population were available for seven out the ten studies included in current meta-analysis (11,12,14,15,17,18,20). Statistically significant heterogeneity was not observed in the primary outcome of clinical success (I2=36%, P=0.16). No difference was observed between ITT patients with AECB receive moxifloxacin versus the comparator (3,860 patients: OR 1.18; 95% CI, 0.98 to 1.42) (Figure 2). Data on treatment success in CE population were reported in nine of the trials (11-18,20). We found no evidence of statistical heterogeneity for clinical success rate in a CE population (I2=0%, P=0.75). Treatment with moxifloxacin was not associated with statistically significant better outcome when compared with other antibiotics in CE population (3,301 patients: OR 1.13; 95% CI, 0.93-1.37) (Figure 3).
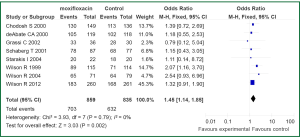
Eight RCTs reported data regarding treatment success in microbiologically evaluable patients (11-13,15-19). No statistically significant heterogeneity was found among the identified studies (I2=0%, P=0.79) (Figure 4). Pooled analysis showed that the use of moxifloxacin was associated with better outcome in ME patients as opposed to control (1,694 patients: OR 1.45; 95% CI, 1.14-1.85). Of the RCTs included in the analysis, five reported data on pathogens isolated at baseline and eradicated. Data on the eradication rates of the three most common pathogens isolated at baseline (i.e., H. influenzae, M. catarrhalis, and S. pneumoniae) were reported in five of the eligible RCTs (11,12,17,19,20). Treatment of ABECB patients with moxifloxacin was associated with higher eradication rates of H. influenzae compared with treatment with comparators (329 isolates, OR 3.48, 95% CI: 1.39-8.73, I2 51%, P=0.07), data from five RCTs (11,12,17,19,20). However, there was no difference between the compared groups on eradication rates of M. catarrhalis (248 isolates, OR 0.61, 95% CI: 0.29-1.27), data from five RCTs (1,12,17,19,20) or of S. pneumoniae (213 isolates, OR 0.80, 95% CI: 0.40-1.57).
Adverse events
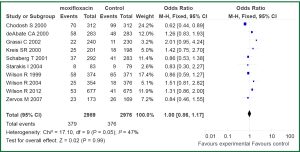
Data on adverse events possibly or probably related to the study medications were reported for all included trials. The most common adverse events included nausea, vomiting, diarrhoea, hypersensitivity, dyspnoea, urticaria and upper abdominal pain. The frequencies of any adverse event were similar for moxifloxacin versus comparator drugs (OR 1.00, 95% CI: 0.86-1.17) (Figure 5).
Publication bias
Upon visual inspection of the funnel plot for the primary outcome, we found evidence of publication bias (absence of small studies in the right lower corner in Figure 6).
Discussion
This systematic review with meta-analysis compared the efficacy and safety of moxifloxacin with that of comparator agents for AECB patients. This meta-analysis indicates that moxifloxacin was associated with similar rates of treatment success and with higher bacteriological success rates compared with comparators (Figures 2,3). The safety analysis regarding the incidence of adverse events proved no difference between the compared treatment arms.
Despite the present evidence suggests that moxifloxacin has a similar efficacy as comparator agents, several unique characteristics make it a superior choice to existing regimens in specific occasions. The results of this study are in agreement with a recent study that was not designed as an RCT. The response to moxifloxacin treatment was broadly independent of the patients’ demographic and disease background. First, moxifloxacin has a broad spectrum of antimicrobial activity, ranging from aerobic to anaerobic, Gram-positive, and Gram-negative bacteria. Moreover, it has an excellent effect against drug-resistant S. pneumoniae, and respiratory Gramnegative pathogens, such as H. influenzae, Mycoplasma pneumoniae, Moraxella catarrhalis, and atypical organisms (i.e., Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella pneumophila). Second, moxifloxacin may mitigate the emergence of resistant strains (22). Studies demonstrated a low spontaneous mutation rate for resistance to moxifloxacin, particularly for Staphylococcus aureus and H. influenzae (23,24). According to the mutant selection window hypothesis, resistant mutants are always selected at antibiotic concentrations above the minimum inhibitory concentration (MIC) but below the mutant prevention concentration (25). Studies determined that the therapeutic concentrations of moxifloxacin lay well above the MPC for S. pneumoniae (26). Third, the once-daily dosing of moxifloxacin may offer benefits compared with regimens that require multiple dosing or combination therapy, including patient convenience and comfort and a lower risk of medication errors. Decreasing the duration of antibiotic courses in respiratory tract infections might contribute to a decrease in these resistance rates. Similarly, the meta-analysis by El Moussaoui et al. demonstrated that a short course of antibiotic treatment is as effective as the traditional longer treatment in patients with mild to moderate AECB and AECOPD (27).
Pathogen eradication has been shown to correlate with improved clinical outcomes and decreased relapse rate. It also contributes to the prevention of emergence and dissemination of resistant pathogens (28,29). Although moxifloxacin provided superior bacterial eradication rates than comparator agents, few studies have demonstrated that treatment with moxifloxacin was associated with a prolonged time to recurrence (30). In the included studies, bacterial load was not determined, so it is possible that, although patients were infected with bacteria, a proportion of them would have an alternative cause of their exacerbation. If the bacterial infection is not the major driver of the clinical manifestations of AECB in all cases then it could be one explanation for the dissociation between clinical and bacteriological outcome would be expected. The present meta-analysis showed no difference between moxifloxacin and the comparator antibiotics for the eradication of M. catarrhalis, and S. pneumoniae. The number of microbiologically evaluable patients was too small to detect differences between them. For patients with AECB due to H. influenzae, moxifloxacin provided superior bacterial eradication rates than s other antimicrobial treatment. Moxifloxacin has excellent role in vitro activity against H. influenzae, that is independent of macrolide resistance mechanisms (31). The predominant bacterial pathogen implicated in AECB and AECOPD is Haemophilus influenzae, which is present in 50% of all bacterial exacerbations, with approximately a further one-third of isolates being either Streptococcus pneumoniae or Moraxella catarrhalis. It should be noted that the causative pathogens of AECB could not be identified in the majority of patients. Thus, correlation of clinical outcomes with bacteriologic outcomes was not possible for most patients.
In terms of safety, no difference was found between compared treatments. Adverse events are usually mild to moderate, in line with the known safety profile of moxifloxacin. A meta-analysis of clinical trial and postmarketing surveillance data for moxifloxacin identified nausea, dizziness, and diarrhea as the most frequent adverse events, which occurred at a rate similar to comparator medications (32,33).
The results of this meta-analysis should be interpreted carefully based on other considerations. First, analysis of any study should critically examine if its endpoints were adequate to demonstrate the potential benefits of the intervention being tested and were clinically relevant. Unfortunately, in the vast majority of antibiotic comparison trials in exacerbations of COPD, end-points used favor the demonstration of equivalence rather than differences among the arms. Clinical studies of antimicrobials in exacerbations of CB such as those performed in the original clinical program have been limited by factors such as inadequate information on patient condition prior to the exacerbation and lack of long-term follow-up, as well as a lack of prospective control for steroid use, which can positively affect the outcome. Second, the ITT principle overlooks the fact that patients may not always receive all their allotted treatment. ITT analysis of noninferiority trials is not conservative, because the inclusion of patients who violate the protocol will tend to minimize differences between study arms, thereby increasing the possibility of results showing noninferiority. Thirdly, a significant proportion of the RCTs included in the meta-analysis allowed the enrolment of patients with an Anthonisen type III ABECB (mild ABECB) (11,12,14) as well as the enrollment of patients without impaired lung function (i.e., without a decrease in FEV1). It may be expected that less significant differences in the effectiveness would be found between different antibiotics. Finally, most of the studies included in the meta-analysis were conducted in the community, even though at least four studies also included hospital inpatients. However, almost all exacerbations were classified as Anthonisen type I or II, we feel some caution is necessary when applying our findings to patients with severe exacerbations who are admitted to hospital with respiratory failure.
Our analysis has several limitations. First, the majority of the RCTs included in current meta-analysis were not designed to focus on long-term outcome, such as exacerbation-free interval or frequency of exacerbations (recurrences) after the resolution of an initial episode of AECB (34). Second, COPD is a heterogenous disease, and acute exacerbations can be of varying severity, partly dependent upon the type of patients in which they occur (35). Most of the studies lack an objective definition of AECB or AECPD. The small number of studies so far does not allow for stratified analysis according to severity of COPD exacerbation. Superiority outcome clinical studies would require considerably larger sample sizes than non-inferiority studies. We should take the heterogeneity of COPD into account, particularly differences in COPD severity, exacerbation frequency and bacterial colonization. In addition, there is heterogeneity in some of the relevant aspects (the patients and comparative drugs included). The clinical effectiveness was assessed at different days in the various RCTs included in the analysis. Trials usually had a primary end point hence after end of treatment we may have missed early relapse due to inadequate treatment. Third, although we extensively searched for relevant studies using multiple databases and multiple search items, and no language restriction was placed on the search, some degree of funnel plot asymmetry suggested the possibility of publication bias. Forth, some patients concomitantly received corticosteroids therapy that could probably have had an impact on the examined outcomes. Finally, the quality of the included studies was not consistent. Some RCTs included in our analysis had major methodological flaws (15,16). Only eight of the included trials were double-blinded. The quality of trials can affect the direction and magnitude of treatment effects when doing a meta-analysis.
Conclusions
In conclusion, despite the limitations of our meta-analysis, we conclude that moxifloxacin has clinical efficacy and microbiological treatment success rates similar to those of comparator drugs in patients with AECB. Moxifloxacin therapy may be a useful alternative to empirical treatment for AECB. Large, well-designed, randomized, multi-center trials warranted to clarify the clinical outcomes (especially long-term outcomes) of patients with AECB receiving moxifloxacin treatment.
Acknowledgements
This research was sponsored by the National Basic Research Program (973 Program) in China (2013CB531402), the Shanghai Subject Chief Scientist Program (07XD14012), Shanghai Leading Talent Projects (No. 036, 2010), and Medical Scientific Research Foundation of Fujian Province (No.2012-CX-23).
Disclosure: The authors declare no conflict of interest.
References
- Seemungal TA, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1418-22. [PubMed]
- Anzueto A. Impact of exacerbations on COPD. Eur Respir Rev 2010;19:113-8. [PubMed]
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007;370:765-73. [PubMed]
- Anthonisen NR, Manfreda J, Warren CP, et al. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987;106:196-204. [PubMed]
- Sethi S. Infectious etiology of acute exacerbations of chronic bronchitis. Chest 2000;117:380S-5S. [PubMed]
- Celli BR, Barnes PJ. Exacerbations of chronic obstructive pulmonary disease. Eur Respir J 2007;29:1224-38. [PubMed]
- Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease (updated 2013) (EB/OL). Available online: http://www.goldcopd.org/guidelines-global-strategy-fordiagnosis-management.html
- Balfour JA, Lamb HM. Moxifloxacin: a review of its clinical potential in the management of community-acquired respiratory tract infections. Drugs 2000;59:115-39. [PubMed]
- Yoshida K, Okimoto N, Kishimoto M, et al. Efficacy and safety of moxifloxacin for community-acquired bacterial pneumonia based on pharmacokinetic analysis. J Infect Chemother 2011;17:678-85. [PubMed]
- Soman A, Honeybourne D, Andrews J, et al. Concentrations of moxifloxacin in serum and pulmonary compartments following a single 400 mg oral dose in patients undergoing fibre-optic bronchoscopy. J Antimicrob Chemother 1999;44:835-8. [PubMed]
- Chodosh S, DeAbate CA, Haverstock D, et al. Short-course moxifloxacin therapy for treatment of acute bacterial exacerbations of chronic bronchitis. The Bronchitis Study Group. Respir Med 2000;94:18-27. [PubMed]
- DeAbate CA, Mathew CP, Warner JH, et al. The safety and efficacy of short course (5-day) moxifloxacin vs. azithromycin in the treatment of patients with acute exacerbation of chronic bronchitis. Respir Med 2000;94:1029-37. [PubMed]
- Grassi C, Casali L, Curti E, et al. Studio Multicentrico con Moxifloxacina nel Trattamento delle Riacutizzazioni de Bronchite Cronica. Efficacy and safety of short course (5-day) moxifloxacin vs 7-day ceftriaxone in the treatment of acute exacerbations of chronic bronchitis (AECB). J Chemother 2002;14:597-608. [PubMed]
- Kreis SR, Herrera N, Golzar N, et al. A comparison of moxifloxacin and azithromycin in the treatment of acute exacerbations of chronic bronchitis. J Clin Outcomes Manag 2000;7:33-7.
- Schaberg T, Ballin I, Huchon G, et al. A multinational, multicentre, non-blinded, randomized study of moxifloxacin oral tablets compared with co-amoxiclav oral tablets in the treatment of acute exacerbation of chronic bronchitis. J Int Med Res 2001;29:314-28. [PubMed]
- Starakis I, Gogos CA, Bassaris H. Five-day moxifloxacin therapy compared with 7-day co-amoxiclav therapy for the treatment of acute exacerbation of chronic bronchitis. Int J Antimicrob Agents 2004;23:129-37. [PubMed]
- Wilson R, Kubin R, Ballin I, et al. Five day moxifloxacin therapy compared with 7 day clarithromycin therapy for the treatment of acute exacerbations of chronic bronchitis. J Antimicrob Chemother 1999;44:501-13. [PubMed]
- Wilson R, Allegra L, Huchon G, et al. Short-term and long-term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitis. Chest 2004;125:953-64. [PubMed]
- Wilson R, Anzueto A, Miravitlles M, et al. Moxifloxacin versus amoxicillin/clavulanic acid in outpatient acute exacerbations of COPD: MAESTRAL results. Eur Respir J 2012;40:17-27. [PubMed]
- Zervos M, Martinez FJ, Amsden GW, et al. Efficacy and safety of 3-day azithromycin versus 5-day moxifloxacin for the treatment of acute bacterial exacerbations of chronic bronchitis. Int J Antimicrob Agents 2007;29:56-61. [PubMed]
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [PubMed]
- Fogarty C, Torres A, Choudhri S, et al. Efficacy of moxifloxacin for treatment of penicillin-, macrolide- and multidrug-resistant Streptococcus pneumoniae in community-acquired pneumonia. Int J Clin Pract 2005;59:1253-9. [PubMed]
- Krasemann C, Meyer J, Tillotson G. Evaluation of the clinical microbiology profile of moxifloxacin. Clin Infect Dis 2001;32 Suppl 1:S51-63. [PubMed]
- Dalhoff A. Comparative in vitro and in vivo activity of the C-8 methoxy quinolone moxifloxacin and the C-8 chlorine quinolone BAY y 3118. Clin Infect Dis 2001;32 Suppl 1:S16-22. [PubMed]
- Drlica K, Zhao X. Mutant selection window hypothesis updated. Clin Infect Dis 2007;44:681-8. [PubMed]
- Blondeau JM, Zhao X, Hansen G, et al. Mutant prevention concentrations of fluoroquinolones for clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother 2001;45:433-8. [PubMed]
- El Moussaoui R, Roede BM, Speelman P, et al. Short-course antibiotic treatment in acute exacerbations of chronic bronchitis and COPD: a meta-analysis of double-blind studies. Thorax 2008;63:415-22. [PubMed]
- Chodosh S, Schreurs A, Siami G, et al. Efficacy of oral ciprofloxacin vs. clarithromycin for treatment of acute bacterial exacerbations of chronic bronchitis. The Bronchitis Study Group. Clin Infect Dis 1998;27:730-8. [PubMed]
- Wilson R, Schentag JJ, Ball P, et al. A comparison of gemifloxacin and clarithromycin in acute exacerbations of chronic bronchitis and long-term clinical outcomes. Clin Ther 2002;24:639-52. [PubMed]
- Chodosh S. Clinical significance of the infection-free interval in the management of acute bacterial exacerbations of chronic bronchitis. Chest 2005;127:2231-6. [PubMed]
- Niederman MS, Anzueto A, Sethi S, et al. Eradication of H. influenzae in AECB: A pooled analysis of moxifloxacin phase III trials compared with macrolide agents. Respir Med 2006;100:1781-90. [PubMed]
- Ball P, Stahlmann R, Kubin R, et al. Safety profile of oral and intravenous moxifloxacin: cumulative data from clinical trials and postmarketing studies. Clin Ther 2004;26:940-50. [PubMed]
- Van Bambeke F, Tulkens PM. Safety profile of the respiratory fluoroquinolone moxifloxacin: comparison with other fluoroquinolones and other antibacterial classes. Drug Saf 2009;32:359-78. [PubMed]
- Chodosh S. Clinical significance of the infection-free interval in the management of acute bacterial exacerbations of chronic bronchitis. Chest 2005;127:2231-6. [PubMed]
- Wilson R, Jones P, Schaberg T, et al. Antibiotic treatment and factors influencing short and long term outcomes of acute exacerbations of chronic bronchitis. Thorax 2006;61:337-42. [PubMed]