Left ventricular assist device exchange for the treatment of HeartMate II pump thrombosis
Introduction
Despite medical successes, left ventricular assist devices (LVADs) continue to have several deficiencies such as infection, bleeding complications, and permanent lifestyle changes for the patient, which need to be considered before implantation (1-7).
With respect to complications in LVAD therapy, pump thrombosis demonstrates the most severe and acute one. Thrombosis most likely occurs due to compliance irregularities with anticoagulation medications, infections which lead to unsteady Coumadin levels, as well as acquired hemostatic disorders (4,8-10). In addition, thrombi might also form as a result of technical difficulties such as surface unevenness of the implanted device.
Because standardized treatment guidelines are missing, pump thrombosis treatment largely varies across centers (11-13). While lysis therapy is widely considered as a first choice, it possesses a high risk of cerebral bleeding as well as embolic stroke. Likewise, a large number of centers are performing LVAD exchange which is expected to treat pump thrombosis. However, this procedure comes at a high risk of thrombus release which may be followed by stroke or bleeding complications.
Within this study, we examine the effects of LVAD exchange for the HeartMate II on the rate of re-thrombosis, complications and outcome of patients.
Methods
Patient selection
Between February 2004 and December 2015 more than 600 LVADs were implanted at our institution. Out of those, we retrospectively studied a patient cohort of 65 patients who underwent LVAD exchange (Figure 1). Forty-one patients underwent LVAD exchange due to pump thrombosis. Out of those 16 HeartMate II patients underwent LVAD exchange for pump thrombosis. Twenty-four patients underwent LVAD exchange due to other reasons such as LVAD infection or device malfunction and were excluded from the study. For the performance of this retrospective study the database of a single center was used. All causes of death and adverse events were determined through retrospective examination of medical records.
LVADs
The LVADs used in this study was the HeartMate II (St. Jude Medical, USA). Other implanted LVADs (e.g., HeartMate 3, Thoratec; MVAD, HVAD HeartWare; HA5, ReliantHeart; other types) and biventricular assist devices and were excluded from the study.
Study design
Data was collected by retrospective electronic medical record review. Endpoints of the study were death, device explantation or heart transplantation. Baseline characteristics were obtained for all patients before LVAD implantation. After LVAD implantation survival results and adverse events were recorded. All causes of death and adverse events were determined through examination of medical records. Because this was a retrospective study, the hospital ethical review board waived the need for patient consent to the study.
Statistical methods
Data collection and analysis were performed retrospectively using IBM SPSS Statistics for Windows Version 23.0 (IBM Corp., Armonk, NY, USA). Categorical and continuous variables were summarized as frequencies, percentages and mean/median with interquartile range, respectively. Paired Student’s t-test and non-parametric Wilcoxon signed-rank test were used for comparisons across groups of continuous variables, respectively. Survival estimates were calculated by the product-limit method of Kaplan-Meier. Differences across groups were quantified using the log-rank test. Two-tailed P≤0.05 were considered significant.
Operative technique
All patients gave informed consent to the procedure. After echocardiographic assessment of the pump position, a lateral thoracotomy was performed to gain access to the pump corpus. Partial rib resection became necessary in half of the cases. After surgical exploration of the old pump position, the venous and the arterial cannulas of the extracorporeal circulation (ECC) were placed in the femoral artery and vein. The outflow cannula of the LVAD was clamped after the onset of the ECC and the device was turned off, the driveline cut and the pump removed from the thorax. After complete removal of the pump housing from the old ring the left ventricular cavity was carefully inspected and thrombi as well as remaining endothelial tissue were carefully removed to prevent suction events.
Preparation and set up of the new pump was executed according to standard instructions for use (IFU) of the company’s protocols.
For HMII, the new pump corpus was connected to the established outflow and inflow cannula. All of the parts of the pump were flushed and de-aired multiple times. Next, the driveline was tunneled through the abdominal wall via standard technique.
After control of surgical hemostasis and inspection of the outflow graft position, a chest tube was placed and the wound was closed. For improved hemostasis during LVAD exchange, all procedures were performed with the use of red blood cells, thrombocytes, fresh frozen plasma and coagulation factors. Intravenous Heparin was started 6 hours postoperatively and administered as a bridge until anticoagulation with phenprocoumon was in the therapeutic range, with a target INR of 2.0–3.0, plus aspirin at 100 mg/day.
Results
Between February 2004 and December 2016, 87 exchanges of LVADs were performed at a single center. Out of those, 41 LVAD exchange were performed due to pump thrombosis (study group). In 24 cases other diagnoses led to LVAD exchange (LVAD infections, technical malfunctions).
In the study group 25 patients (61%) were supported by HeartWare and 16 patients (39%) were supported by HeartMate II.
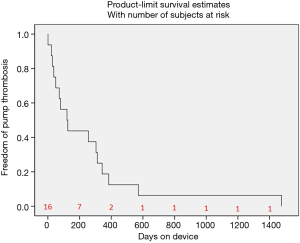
A total of 9 patient years (3,272 days) were analyzed. Mean days on device until the occurrence of pump thrombosis was 261 days with a minimum of 1 and a maximum of 1,475 days on device (Figure 2).
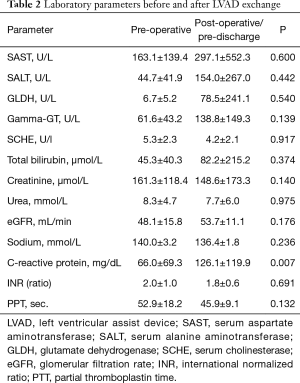
Detailed baseline characteristics of the study group can be found in Table 1. Preoperative laboratory values are listed in Table 2.

Full table
Full table
In nine patients of the study group (56%) dilative cardiomyopathy was the primary heart disease prior to LVAD implantation. The majority of patients were INTERMACS level III. 0 patients of the study group (0%) were initially classified as NYHA IV prior to LVAD implantation.
All patients showed flow irregularities and increase of power. 26 patients presented with hematuria (63%) out of which 1 patient (6.3%) needed preoperative dialysis.
Average ICU stay was 4.2±6.2 days and average in-hospital stay 35.7±50.8 days after LVAD exchange.
Outcomes after LVAD exchange due to pump thrombosis
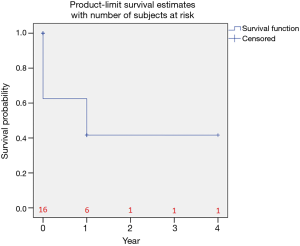
The Kaplan-Meier curve in Figure 3 illustrates the patient survival. After thirty days the survival rate was 75.0%, 75.0% after six months and 62.5% one year after LVAD exchange.
Out of the study cohort, two patients have successfully undergone heart transplantation on postoperative day (POD) 102 and 140. One patient underwent successful LVAD explantation due to myocardial recovery on POD 248.
A total of six patients died during the complete one year follow up of this study (29%). Two patients died in the second year after LVAD exchange. The remaining five patients are still ongoing on the device. The longest ongoing patient out after LVAD exchange because of pump thrombosis is on device for more than 7 years as of April 1st, 2017.
Re-operation
Out of the study group a total of 4 patients (25%) underwent a second LVAD exchange.
Two patients underwent a second LVAD exchange because of recurrent pump thrombosis on POD 0 and 133 respectively. Two patients underwent a second LVAD exchange because of device infection on POD 409 and 566.
Causes of death
One year after LVAD exchange, out of 16 patients, 6 patients had died (38%). Main cause of death was multi-organ failure with 5 deaths on POD 1, 15, 18, 204 and 321 respectively. One patient died because of sepsis on POD 4.
Complications
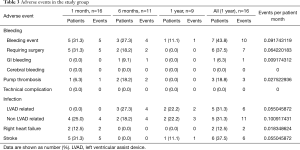
Complications and adverse events are listed in Table 3. In the first month after LVAD exchange, five strokes occurred in the study group (31.3%) and one patient presented re-pump thrombosis (6.3%). Five patients required (31.3%) re-operation because of bleeding events. Two patients presented right heart failure (12.5%) and four patients presented with infections, out of which four were non LVAD-related. Dialysis was needed in 4 patients postoperatively (25.0%).
Full table
Six months after LVAD exchange one presented with GI bleedings (9.1%). Two patients suffered from recurrent pump thrombosis.
One year after LVAD exchange, 14 patients underwent re-exchange due to pump thrombosis (34%). Eight patients suffered from a LVAD related infection out of which two patients were treated by pump exchange. Twelve patients suffered a stroke postoperatively (29%). No technical complications of the VAD were recorded in the study group.
Lactate dehydrogenase (LDH) and free hemoglobin (fHb)
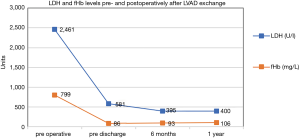
The LDH (U/L) as well as fHb (mg/L) laboratory values in the course of LVAD pump thrombosis are displayed in Figure 4. The fHb as well as the LDH curves show a steep decline. After the sixth month, the curve is decreasing to normal values. LDH levels showed a decrease during the whole study period. With an average LDH value of 395.4 U/L and an average fHb of 93.5 mg/L six months after LVAD exchange. After 1-year average LDH level was 400.2 U/L and average fHb level was 106.3 mg/L respectively.
Discussion
Pump thrombosis is the most severe and acute complication of LVAD therapy with an emergent need for therapy which has been extensively discussed in the past years. For HeartMate II, Starling et al. reported that pump exchange or death due to pump thrombosis increased during 2011 and 2012, but the level of increase remained small (14-18). A risk factor analysis suggested that a number of patient-related factors contribute to the risk of thrombosis (15). While other centers reported increases thrombosis rates, others reported a steady rate of 2.2% (16). Smedira et al. analyzed a total of 995 thrombosed pumps and also reported an increase in pump thrombosis in 2010, which reached a maximum in 2012, and then plateaued at a level that was reportedly 3 times higher than pre-2010 (17).
Due to the limited therapeutic options, prevention of the development of pump thrombosis is of highest importance. The angle of the inflow cannula plays an important role in thrombus formation by creating un-physiological blood flow patterns (18). High pump speeds ≥9,000 RPMs for the HMII are associated with a lower risk for pump thrombosis according to Maltais et al. (19). Diligent patient education about the use of anticoagulation and strict monitoring (e.g., via CoaguCheck®) improve therapy safety. A recent study suggests a higher pump thrombosis risk for patients BMI >30 kg/m2. Therefore, weight reduction for obese patients should be essential (20). Furthermore, high blood pressure is associated with an increased risk of pump thrombosis (10).
Since atrial fibrillation is a risk factor for thrombus formation and is often associated with a high risk of stroke, a number of studies examined the effects of cardiac arrhythmias on LVAD therapy. Xuereb et al. report no significant effect of preoperative atrial fibrillation on postoperative rates of pump thrombosis or stroke (21). Stulak et al. even showed that Patients with preoperative AF have a lower freedom from thromboembolic events after LVAD implantation (22). Enriquez et al. examined 106 HMII patients and differentiated between paroxysmal atrial fibrillation (PAF) and persistent atrial fibrillation (PeAF). They concluded that although PAF is not associated with worse outcome, PeAF may be associated with increased mortality and re-hospitalizations (23). Concomitant cryoablation for tachycardias combined with LVAD implantation has been described before (24) and might be a solution to reduce the risk of pump thrombosis in patients with AF.
Anticoagulation is a major factor in preventing pump thrombosis. Yet, too strict regiments increase the risk of bleeding complications such as GI bleeding or stroke (25). The TRACE study questioned the need of double anti-coagulation in HMII patients to reduce the risk of pump thrombosis. The 1-year results revealed that reducing anti-thrombotic therapies in response to bleeding among HMII patients was achievable but may be associated with a higher risk for device thrombosis (8,9). The recently published 2 years results of this study showed that managing HMII patients with a vitamin K antagonist with a target international normalized ratio of 2.3 without antiplatelet therapy may help to reduce the incidence of major bleeding without increasing the risk of thromboembolic events, including ischemic stroke and pump thrombosis (9).
Koene et al. showed that following HeartMate II implantation HAS-BLED and CHA2DS2-VASc scores of ≥3 conferred significantly higher risks of bleeding and thromboembolic events, respectively (25). Also, a newly developed left ventricular dimension decrement index at optimized speed setting on pre-discharge echocardiography is associated with LVAD thrombosis (26). Therefore, these scores might be useful to detect patients with a high risk of pump thrombosis.
Even though the need for LVAD exchange is a hazardous complication in VAD therapy, it can be used to upgrade a patient to a new generation assist device e.g., HeartMate 3 (27-30). The exchange procedure offers an opportunity to upgrade patients to a new generation pump which offers e.g., advanced reduction of adverse events or longer battery capacities (27). New generation pumps such as the HeartMate 3 trials reported no pump thrombosis in the CE mark study as well as in the U.S. based MOMENTUM study. Debilitating strokes were reported in 8% of the patients in the CE mark study and in 7.9% of the patients in the MOMENTUM trial (31,32). Consequently, exchange to a new generation device should be considered when exchanging an LVAD for pump thrombosis.
Generally, LVAD exchanges are associated with high risk of stroke or air embolism as seen in a perioperative stroke rate of 29% in this study. Therefore, we highly recommend using on-pump techniques in the majority of these cases (33,34). Using the on-pump techniques offers the opportunity to inspect and remove endothelial tissue as well as thrombotic material from the left ventricle (35).
To conclude, the treatment and the prevention of pump thrombosis remain challenging. In general, the question of risk factors for thrombus formation as well as treatment options need to be further assessed with controlled studies and increased patient populations.
Limitations of the study
This study has some limitations. The data is retrospectively collected and analyzed and therefore it is subject to the limitations associated with retrospective studies. All exchange procedures were performed at one institution and therefore generalizability may be limited and affected by institutional experience. The results of surgical studies are prone to learning curves and single center’s specific characteristics. Moreover, the study period began several years ago. Therapeutic strategies and increased clinical experience might have improved today’s results. Additionally, the number of patients with pump thrombosis was small, which reduces the statistical power and ability to infer positive findings. As such, larger studies need to be done to further study the outcomes after LVAD exchange due to pump thrombosis.
Conclusions
It is generally feasible to treat pump thrombosis of the HeartMate II via LVAD exchange. Yet, the exchange procedure is not without risk and the risk of re-thrombosis, stroke, postoperative dialysis and perioperative complications remains high.
Acknowledgements
None.
Footnote
Conflicts of Interest: JD Schmitto receives consultation fees from Thoratec Cooperation. JD Schmitto and G Dogan are consultants for HeartWare Cooperation. The other authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the hospital ethical review board and the hospital ethical review board waived the need for patient consent to the study.
References
- Hanke JS, Rojas SV, Avsar M, et al. Minimally-invasive LVAD implantation: State of the art. Curr Cardiol Rev 2015;11:246-51. [Crossref] [PubMed]
- Stehlik J, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for heart and lung transplantation: twenty-eighth adult heart transplant report—2011. J Heart Lung Transplant 2011;30:1078-94. [Crossref] [PubMed]
- Modica M, Ferratini M, Torri A, et al. Quality of life and emotional distress early after left ventricular assist device implant: a mixed-method study. Artif Organs 2015;39:220-7. [Crossref] [PubMed]
- Goldstein DJ, Naftel D, Holman W, et al. Continuous-flow devices and percutaneous site infections: clinical outcomes. J Heart Lung Transplant 2012;31:1151-7. [Crossref] [PubMed]
- Cagliostro B, Levin AP, Fried J, et al. Continuous-flow left ventricular assist devices and usefulness of a standardized strategy to reduce drive-line infections. J Heart Lung Transplant 2016;35:108-14. [Crossref] [PubMed]
- Nienaber JJ, Kusne S, Riaz T, et al. Clinical manifestations and management of left ventricular assist device-associated infections. Clin Infect Dis 2013;57:1438-48. [Crossref] [PubMed]
- Harvey L, Holley C, Cogswell R, et al. Driveline infection after HeartMate II associated with lower rates of cardiac transplantation and increased incidence of sepsis in bridge-to-transplant population. Minn Med 2014;97:40. [PubMed]
- Katz JN, Adamson RM, John R, et al. Safety of reduced anti-thrombotic strategies in HeartMate II patients: A one-year analysis of the US-TRACE Study. J Heart Lung Transplant 2015;34:1542-8. [Crossref] [PubMed]
- Netuka I, Litzler PY, Berchtold-Herz M, et al. Outcomes in HeartMate II patients with no antiplatelet therapy: 2-year results from the European TRACE Study. Ann Thorac Surg 2017;103:1262-8. [Crossref] [PubMed]
- Najjar SS, Slaughter MS, Pagani FD, et al. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant 2014;33:23-34. [Crossref] [PubMed]
- Schubmehl HB, Tchantchaleishvili V, Storoznsky E, et al. Echocardiographic detection of left ventricular thrombus in patients undergoing HeartMate II left ventricular assist device implantation. Int J Artif Organs 2016;39:491-6. [Crossref] [PubMed]
- Tran BC, Nijjar PS. Role of contrast CT for the diagnosis and the prognosis of suspected LVAD thrombosis. J Card Surg 2017;32:162-5. [Crossref] [PubMed]
- Hubbert L, Sundbom P, Loebe M, et al. Acoustic analysis of a mechanical circulatory support. Artif Organs 2014;38:593-8. [Crossref] [PubMed]
- Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med 2014;370:33-40. [Crossref] [PubMed]
- Kirklin JK, Naftel DC, Pagani FD, et al. Pump thrombosis in the Thoratec HeartMate II device: An update analysis of the INTERMACS Registry. J Heart Lung Transplant 2015;34:1515-26. [Crossref] [PubMed]
- Schmitto JD, Avsar M, Haverich A. Increase in left ventricular assist device thrombosis. N Engl J Med 2014;370:1463-4. [Crossref] [PubMed]
- Smedira NG, Blackstone EH, Ehrlinger J, et al. Current risks of HeartMate II pump thrombosis: Non-parametric analysis of Interagency Registry for Mechanically Assisted Circulatory Support data. J Heart Lung Transplant 2015;34:1527-34. [Crossref] [PubMed]
- Aliseda A, Chivukula VK, Mcgah P, et al. LVAD Outflow Graft Angle and Thrombosis Risk. ASAIO J 2017;63:14-23. [Crossref] [PubMed]
- Maltais S, Kilic A, Nathan S, et al. PREVENtion of HeartMate II Pump thrombosis through clinical management: the PREVENT multi-center study. J Heart Lung Transplant 2017;36:1-12. [Crossref] [PubMed]
- Han JJ, Sooppan R, Johnson AP, et al. Higher body mass index increases risk of HeartMate II pump thrombosis but does not adversely affect long-term survival. Circ J 2017;81:213-9. [Crossref] [PubMed]
- Xuereb L, Go PH, Kaur B, et al. Impact of preoperative atrial fibrillation on postoperative thromboembolic events after left ventricular assist device implantation. Ann Thorac Surg 2016;102:1543-9. [Crossref] [PubMed]
- Stulak JM, Deo S, Schirger J, et al. Preoperative atrial fibrillation increases risk of thromboembolic events after left ventricular assist device implantation. Ann Thorac Surg 2013;96:2161-7. [Crossref] [PubMed]
- Enriquez AD, Calenda B, Gandhi PU, et al. Clinical impact of atrial fibrillation in patients with the HeartMate II left ventricular assist device. J Am Coll Cardiol 2014;64:1883-90. [Crossref] [PubMed]
- McIlvennan CK, Babu AN, Brieke A, et al. Concomitant surgical cryoablation for refractory ventricular tachycardia and left ventricular assist device placement: a dual remedy but a recipe for thrombosis? J Cardiothorac Surg 2016;11:53. [Crossref] [PubMed]
- Koene RJ, Win S, Naksuk N, et al. HAS-BLED and CHA2DS2-VASc scores as predictors of bleeding and thrombotic risk after continuous-flow ventricular assist device implantation. J Card Fail 2014;20:800-7. [Crossref] [PubMed]
- Joyce E, Stewart GC, Hickey M, et al. Left ventricular dimension decrement index early after axial flow assist device implantation: A novel risk marker for late pump thrombosis. J Heart Lung Transplant 2015;34:1561-9. [Crossref] [PubMed]
- Schmitto JD, Hanke JS, Rojas SV, et al. First implantation in man of a new magnetically levitated left ventricular assist device (HeartMate III). J Heart Lung Transplant 2015;34:858-60. [Crossref] [PubMed]
- Hanke JS, Haverich A, Schmitto JD. Exchange of a HeartWare HVAD to a HeartMate 3 left ventricular assist device. J Heart Lung Transplant 2017;36:480-1. [Crossref] [PubMed]
- Hanke JS, Haverich A, Schmitto JD. Exchange of a HeartMate II left ventricular assist device with a HeartMate 3 pump. J Heart Lung Transplant 2016;35:944-6. [Crossref] [PubMed]
- Hanke JS, Rojas SV, Dogan G, et al. First series of left ventricular assist device exchanges to HeartMate 3. Eur J Cardiothorac Surg 2017;51:887-92. [Crossref] [PubMed]
- Netuka I, Sood P, Pya Y, et al. Fully magnetically levitated left ventricular assist system for treating advanced HF: a multicenter study. J Am Coll Cardiol 2015;66:2579-89. [Crossref] [PubMed]
- Mehra MR, Naka Y, Uriel N, et al. A fully magnetically levitated circulatory pump for advanced heart failure. N Engl J Med 2017;376:440-50. [Crossref] [PubMed]
- Rojas SV, Avsar M, Khalpey Z, et al. Minimally invasive off-pump left ventricular assist device exchange: anterolateral thoracotomy. Artif Organs 2014;38:539-42. [Crossref] [PubMed]
- Rojas SV, Haverich A, Schmitto JD. off-pump versus on-pump left ventricular assist device exchange. Artif organs 2014;38:992. [Crossref] [PubMed]
- Scandroglio AM, Kaufmann F, Pieri M, et al. Diagnosis and treatment algorithm for blood flow obstructions in patients with left ventricular assist device. J Am Coll Cardiol 2016;67:2758-68. [Crossref] [PubMed]