Single-stage endovascular management of complicated thoracic aorta coarctation concurrent with aortic arch aneurysm using a novel fenestration device
Introduction
Coarctation of the aorta (COA) is a congenital pathology, with a morbidity rate of 0.004% in neonatal cohorts. COA takes up for 5–7% of all congenital heart disease (1), and 17% of COA are accompanied by aorta aneurysm (2). The surgical treatment for COA has been introduced since 1940s and Alexander et al. (3) first reported aortectomy for COA. In 1948, Harris et al. (4) performed excision and End-to-End suture of aorta for coarctation and aneurysm of the aorta. The present available modality for treating COA is classified as open surgery, hybrid procedure and endovascular therapy, etc. (5). However, as to a rare case with COA complicated by aneurysm of aortic arch and its branches, the former two algorithms has been indicated as a treatment of choice as evidenced by current guideline (6-9). Endovascular reconstruction of supraaortic branches coexisting with COA has not been reported according to current medical library. Here we reported a case with COA concurrent with aortic arch aneurysm invading left subclavian artery (LSA) and successfully performed endovascular exclusion of aneurysm, coarctation and simultaneous fenestration of left common carotid artery (LCCA) using a novel fenestration device.
Technique
A 46-year-old female presented with a mass in thyroid region and through initial computed tomography (CT) an aortic arch aneurysm was observed. For further confirmation of pathology she underwent CT angiography (CTA), which revealed that an enormous irregular aneurysm was located at aortic arch. Aneurysm body had a maximal diameter of 86.5 mm and it was 10 mm distal to LCCA. LSA arose from the aortic arch aneurysm and had a severe tortuosity and dilatation. The distal exit end of aneurysm had a coarctation with a minimal diameter of 2.7 mm (Figure 1A,B,C). The patient had a long history of high blood pressure (BP) with systolic value 170 mmHg and the D-value between superior and lower extremity BP was 40 mmHg. Cardiac and pulmonary function test showed that no obvious abnormality was found. Based upon the confirmed diagnosis, surgical indications and patient’s desire to have a endovascular reconstruction, we prepared to perform percutaneous transluminal angioplasty (PTA) with placement of Cheatham Platinum (CP) stent prepared and simultaneously implement endovascular exclusion of aneurysm and in situ fenestrate LCCA.
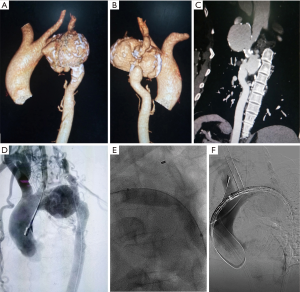
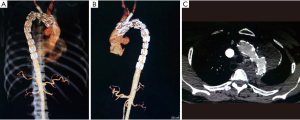
Under general anesthesia, the patient was positioned supine and right femoral artery was punctured using Selindler technique. Afterwards 2 Proglide vascular anastomosis devices (Perclose ProGlide, Abbott Inc., USA) were pre-positioned and 12-French short sheath were introduced. A 7-French short sheath was then interpositioned into LCCA, after which a 6F 55 mm renal artery sheath (Flexor® Check-Flo® Introducer, COOK Inc., USA) was introduced into left brachial artery. Through preserved sheath in carotid artery, a pigtail catheter was advanced into ascending aorta and an initial angiogram was performed. The aortography demonstrated the same results as preoperative CTA (Figure 1D). Intraoperative BP supervision indicated a pressure gradient of 50 mmHg across the coarcted segment of aorta. Via femoral or carotid artery access, 0.035 inch guidewire in coordination with 5 French single-curved or 0.035 inch supporting catheter (Support Catheter Rubicon tm 35, Boston scientific, USA) has been introduced but could not advance across aneurysm cavity after multiple endeavors. After that a trapper were introduced via femoral access and successfully capture the 0.035 inch soft guidewire followed by pulling it outside the sheath. Then a 5 French single-curved catheter was advanced into ascending aorta through the indicated guidewire and exchanged with Lunderquist super stiff guidewire. Immediately following that, an initial 6 mm × 40 mm and then 12 mm × 40 mm balloon (Ballon Dilatation Catheter Mustang tm, Boston scientific, USA) were inserted to dilatate the coarctation lesion (Figure 1E). Then a 36 mm × 200 mm covered stent (XJZDZ32200, Ankura® , Lifetech Inc., China) was implanted and completely deployed until the proximal end of endograft was positioned at the right side of innominate artery (IA), during which systolic BP is controlled to 110 mmHg. After the systolic BP was elevated to 160 mmHg, the novel fenestration device (Quick-Fenestrater) were inserted through preserved sheath into carotid artery and made a contact with the endograft. Then the stabilizing stent at front end was released and fenestrating needle are manipulated to swiftly create a hole at the stent graft. Inside the needle lumen, a 0.035 inch soft guidewire was transported into ascending aorta. Through the wire, the stabilizing stent was recollected and Quick-Fenestrater was pushed forwards and the cephalic needle was applied twice to enlarge the fenestration (Figure 2A,B,C,D). Thereafter the fenestration device was pulled back and exchanged with 4 mm × 20 mm balloon (Ballon Dilatation Catheter Mustang tm, Boston scientific, USA) to dilatate the fenestration. Across the fenestration, an 8 mm × 37 mm balloon-dilated stent (Express tm LD Vascular, Boston scientific, USA) was interpositioned to revascularize LCCA. A aortography was performed at this time and revealed a precise location of aortograft, an exclusion of aneurysm, a patent LCCA and a eradication of COA. Then a balloon (AB46, Reliant, Medtronic Inc., USA) was introduced through Lunderquist super stiff guidewire via femoral access to once again dilatate the narrowest lesion of aorta (Figure 2E). The patient had an immediate reduction of BP from 170 to 130 mmHg, with residual gradient across the coarctation decreasing to 10 mmHg. With regard to the lesioned LSA, we abandoned in situ fenestration (ISF) for it because of the severe tortuosity of LSA and its original site at the aneurysm as evidenced by angiogram through sheath inserted into left brachial artery. A final aortogram was made and demonstrated that the coarctation was eradicated, a complete exclusion of aneurysm with no endoleak occurring and IA and LCCA were patent (Figure 2F), therefore a CP stent was not utilized. The intraoperative and postoperative stages are uneventful with no complication observed for patient and 6-month follow-up CTA displayed desirable outcome (Figure 3A,B,C).
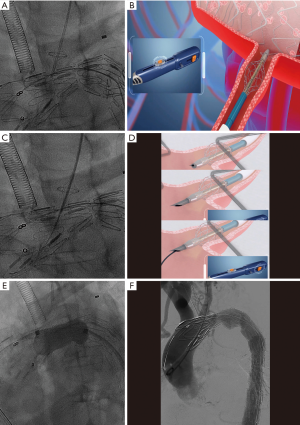
Quick-Fenestrater
Quick-Fenestrater was developed by Shanghai Changzheng Hospital with patent right (201110237935.8) acquired in cooperation with Luoyin Medical Apparatus Company in Suzhou. The device was specialized in fenestrating supraaortic branches in situ during thoracic endovascular aortic repair (TEVAR). The main principle of it is classified as: (I) through the supporting stent at fore-end of the device, the needle was targeted perpendicular to the graft material and the puncture site was stabilized in the central point of the covered branch; (II) the needle was controllable in strength and motivation and can be utilized to quickly perform fenestration; (III) guidewire can advance inside the needle and effectively introduce balloon to accomplish the fenestration.
Discussion
Aortic coarctation, as a congenital cardiovascular anomaly, may be associated with serious pathologies such as aneurysms. Patients can be asymptomatic in their early age, but coarctation may cause systemic refractory hypertension and other malperfusion-related disease such as coronary artery disease, myocardial infarction, the emerge of which predicts that surgical intervention is required. If the coarctation is combined with aneurysm in continual growth, which carries a high risk of rupture or causes compression symptom, surgical procedure is emergent and beneficial. The surgical reconstruction of COA was initially described in 1944, and until now, several other surgical techniques have been developed to treat child and adult patients. The recommended procedure is subgrouped into three components: open surgery, hybrid surgery and endovascular intervention. Open surgery, including end-to-end anastomosis with resection of coarctation, prosthetic patch aortoplasty, graft vascular bypass, etc., can bring patients a good long term results. However, they were gradually replaced by endovascular treatment due to the surgical injuries, difficulties of the surgery and high mortality rate. PTA, stent angioplasty has been widely utilized to treat patients with COA since the instruments are greatly developed and modified (10). Presently, CP stent was designed to be used in the COA and the safety and effectiveness of CP stent for selected patients have been illustrated in several clinical trials (11,12). The aneurysm can be located in the pre- or post-coarctation site, the mechanism underling which is uncertain and may be related to atherosclerosis, intimal-media histochemical degeneration, hemodynamic changes caused by coarctation, etc. As to the coarctation with small aneurysm, the two lesions can be disposed simultaneously with surgical treatment. Nonetheless, the case with aortic coarctation combined with aneurysm at aortic arch and its branches is extremely rare and the selection of surgical plan remains to be unconfirmed. Only a few case reports has indicated resection of pathologies combined with aortic arch replacement, supraaortic vascular reconstruction combined with prosthetic graft transposition, two-stage procedure including first debranching for revascularizing supraaortic vessels and then covered endograft interposition, etc., as a surgical alternative for treating such complex conditions (6-9).
The case reported here is extremely rare. The lesion characterization lies in that: (I) the aneurysm was enormous, irregular, calcified, located at the aortic arch, around which the aneurysm was forming into a dumbbell shape; (II) the aneurysm was merely 10 mm distant to the LCCA; (III) LSA arose from the aneurysm and had a severe distortion and dilatation; (IV) the narrowest site is at the central part of the dumbbell-shaped aneurysm with a diameter of 2.7 mm and the coarctation segment of aorta is severe calcified. Above-mentioned trait of the lesion poses great difficulties for surgical intervention. Through detailed retrieval, no treatment has been recommended for such rare case. The aforementioned modalities could be applied, but the patient had an intense desire for endovascular therapy. The difficulties in performing endovascular procedures depend on the lesion feature, one of which is how to dispose with the aneurysm and coarctation and the sequential managing order of surgical procedure. Considering that TEVAR is extensively applied to handle with aortic aneurysm cases and deployment of CP stent has been proved as a safe, effective alternative for aortic coarctation, we selected to use endovascular management. Initially, the matter whether to first use covered stents to treat aneurysms or first use CP stent to dispose of coarctation should be discretely concerned. As evaluated by preoperative image, it may be hard for the covered stent to get through the narrowest site, which is evidenced by the difficulties of 5F catheter advancing across the coarctation after guidewire passes through the lesion during procedure. However, if we prepared to dispose the coarctation first, the deployment of CP stent may greatly add up to the risk of aorta rupture due to the severe stenosis and calcification of the aorta (11). Comparing to CP stent, initial introduction of covered aortic endograft can protect the aorta and to some extent eradicate the coarctation contributed by the intense self-expandability of covered stent. Finally in the principle of gradually dilatation stenotic lesions, we first used small-diameter high-pressure balloon to expand the stenosis so that the covered stent could smoothly go through. Then a large-diameter balloon was used for post-dilatation of endograft to sufficiently enlarge the stenosis. After that the BP gradient was significantly reduced as indicated by intraoperative BP survey. What is more, the landing zone of covered stent and the reconstruction of supraaortic branches remain to be investigated in this case. For sufficient landing area, the proximal end of stent had to be positioned at the left side of IA and the endograft had to cover the LCCA and LSA because the aneurysm is 10 mm distant to LCCA, which could not offer a landing zone of 20 mm for the stent. For acquiring enough landing zone, the present methods to preserve blood perfusion into supraarch artery included debranching, chimney technique, etc. These modalities may create some defects, such as huge trauma, endoleak, extended hospitalization, etc. ISF was first reported by McWilliams et al. (13) in year 2004 and has been now indicated as a safe, effective procedure for recanalization of supraaortic arch during TEVAR (14). This technique may be adequate for the fenestration of LCCA in the indicated case. Nevertheless, the lack of related fenestration instrument greatly limits the application of ISF in recent 10 years or so. Based upon the acquired experience in performing ISF, we devised a specialized apparatus by which ISF for supraaortic arteries could be made during TEVAR. The working model for this device is divided by three steps: (I) through the supporting stent at the front end, the endograft materials covering the branch is stabilized to make sure that the fenestration site is at the central point; (II) being controllable in strength, the needle is pushed forwards to quickly fenestrate the endograft and can avoid advancing too ahead; (III) going through the needle lumen, a guidewire is inserted and fast accomplish the fenestration. In the experiments in vitro, we have performed ISF using this device in 8 white pig (median weight 60±1.8 kg) with desirable results. The above-mentioned experiment primarily demonstrated that the fenestrating device is controllable and highly effective for fenestration, furthermore, it can bring about less vascular injuries. The device was nominated “Quick-Fenestrater” by our center. We have acquired the approval from the ethnical commission in the author’s affiliation: Shanghai Changzheng Hospital and put this device into clinical trials, of which the reported case here is the first experiment. In the procedure of ISF, 3 seconds was spend for fenestrating the endograft materials and 4 minutes for the entire procedure. The current most commonly used instruments for ISF are laser or homemade mechanistic needle. The former is invisible under fluoroscopy and requires sensation and constant endeavors. It may cause heat injuries to adjacent vessels. Homemade needle, uncontrollable in strength and directional regulation, may also cause harm to surrounding tissue (14). Although current research has indicated that laser or mechanistic needle-guided procedure is effective during ISF for multiple supraaortic arteries (15), the case cohort is small so that the safety of these processes cannot be guaranteed. On the other hand, to substantiate whether Quick-Fenestrater can be a safer, more effective and efficient alternative than other traditional device for performing ISF to revascularize multiple supraaortic branches during TEVAR necessitates further clinical research.
Conclusions
Aortic coarctation combined with aneurysm, as an extremely rare clinical entity, are heterogeneous lesions which may vary in clinical symptoms, lesion location and poses a great difficulty for endovascular treatment. As primarily indicated by the reported case, an integrated algorithm of PTA, TEVAR, CP stent implantation and ISF could be performed to treat the complicated case with elevated technique success rate, reduced complication frequency and shortened recovery intervals. Quick-Fenestrater can be adaptable to such complex disease and may be indicated as a safe, effective, less time-consuming modality for the reconstruction of covered artery. However, investigation of the endovascular therapy of COA combined with aneurysm in larger populations using Quick-Fenestrater should be further conducted.
Acknowledgements
Funding: This work was supported by the Specifically Invited Professor of Oriental Scholar of Shanghai Colleges and Universities Tracking Program (GZ2016008), the Guiding Project of Western medicine from the Science and Technology Commission of Shanghai Municipality (16411966500), and grants from the National Natural Science Foundation of China (8157020854). Shanghai Municipal Commission of Health and Family Planning Research project (20164Y0096).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: The study was approved by ethics committee of Shanghai Changzheng Hospital (No. 2017SL038). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Allen BD, Barker AJ, Gabbour M, et al. Aortic coarctation augments changes in thoracic aortic hemodynamics in pediatric and young adult patients with bicuspid aortic valve. J Cardiovasc Magn Reson 2013;15:300. [Crossref]
- Karabulut H, Senay S, Toraman F, et al. Primary endovascular treatment of native thoracic aortic aneurysm associated with coarctation. Ejves Extra 2007;14:36-8. [Crossref]
- Alexander J, Byron FX. Aortectomy for thoracic aneurysm. JAMA 1944;126:1139-1145. [Crossref]
- Shumacker HB. Coarctation and Aneurysm of the Aorta: Report of a Case Treated by Excision and End-to-End Suture of Aorta. Ann Surg 1948;127:655-65. [Crossref]
- Torok RD, Campbell MJ, Fleming GA, et al. Coarctation of the aorta: Management from infancy to adulthood. World J Cardiol 2015;7:765-75. [Crossref] [PubMed]
- Gunn J, Cleveland T, Gaines P. Covered stent to treat co-existent coarctation and aneurysm of the aorta in a young man. Heart 1999;82:351. [Crossref] [PubMed]
- Pogorzelski R, Wołoszko T, Toutounchi S, et al. Intravascular Treatment of Left Subclavian Artery Aneurysm Coexisting with Aortic Coarctation in an Adult Patient. Open Med (Wars) 2017;12:1-4. [Crossref] [PubMed]
- Zhou W, Zhou W, Qiu J, et al. Hybrid procedure to treat aortic arch aneurysm combined with aortic arch coarctation and left internal carotid artery aneurysm (Case Report). J Cardiothorac Surg 2014;9:3. [Crossref] [PubMed]
- Teimouri H, Sabzi F, Dabiri S. Congenital saccular aneurysm of coarctation of aorta: a case report. J Tehran Heart Cent 2013;8:210-2. [PubMed]
- Torok RD, Campbell MJ, Fleming GA, et al. Coarctation of the aorta: Management from infancy to adulthood. World J Cardiol 2015;7:765-75. [Crossref] [PubMed]
- Ewert P, Schubert S, Peters B, et al. The CP stent--short, long, covered--for the treatment of aortic coarctation, stenosis of pulmonary arteries and caval veins, and Fontan anastomosis in children and adults: an evaluation of 60 stents in 53 patients. Heart 2005;91:948-53. [Crossref] [PubMed]
- Tan YL, Chih WL, Wang JK, et al. Paradoxical Hypertension after Successful Cheatham Platinum Stent Implantation in an Adolescent with Coarctation of the Aorta. Acta Cardiol Sin 2016;32:755-7. [PubMed]
- McWilliams RG, Murphy M, Hartley D, et al. In situ stent-graft fenestration to preserve the left subclavian artery. J Endovasc Ther 2004;11:170-4. [Crossref] [PubMed]
- Crawford SA, Sanford RM, Forbes TL, et al. Clinical outcomes and material properties of in situ fenestration of endovascular stent grafts. J Vasc Surg 2016;64:244-50. [Crossref] [PubMed]
- Qin J, Zhao Z, Wang R, et al. In Situ Laser Fenestration Is a Feasible Method for Revascularization of Aortic Arch During Thoracic Endovascular Aortic Repair. J Am Heart Assoc 2017;6:e004542. [Crossref] [PubMed]


