A study of weekly docetaxel and carboplatin as first-line chemotherapy for advanced non-small cell lung cancer
Introduction
Throughout the world, most of the cancer-related deaths are resulted from lung cancer, among which non-small cell lung cancer (NSCLC) holds a constitution of approximately 80% of all cases (1-3). And locally advanced inoperable (stage IIIB) or metastatic (stage IV) disease influences almost 70% of the patients with NSCLC (4). The combinations of platinum and some third-generation new drugs, such as taxanes, gemcitabine and vinorelbine have been regarded as the standard treatment for advanced NSCLC (5), which provides a better survival advantage over the best supportive care alone in patients with a good Eastern Cooperative Oncology Group (ECOG) performance status (PS) (6-10).
The combination of docetaxel with cisplatin, exerts a significant effect on NSCLC, with response rate (RR) ranging from 35% to 45%, and overall median survival reaching 12 months (9). However, the clinical use of cisplatin is sometimes hampered by its severe toxicity, such as nephrotoxicity, gastrointestinal toxicity including nausea and vomiting, and neurotoxicity which requires hydration to release renal damage. Carboplatin, a second-generation platinum-containing compound, is much less nephrotoxic, neurotoxic and emetogenic than its parent compound cisplatin. A recent meta-analysis for advanced NSCLC reported that the rate of ≥ grade 3 nausea and vomiting for cisplatin-based therapy was higher than carboplatin-based therapy (11,12). And, although cisplatin-based chemotherapy produced a higher RR, it brought no improvement in survival compared with carboplatin-based therapy. So, carboplatin may be a good alternative choice for NSCLC.
The traditional 3-weekly schedule of docetaxel is 75 mg/m2 afflicted by severe grade myelotoxicity (13,14), for example 12% to 16% patients were observed experiencing with febrile, which had been a major limitation, particularly in the elder patients, and patients with a poor PS. A weekly docetaxel, been studied as a single agent in second line NSCLC, improved the toxicity profile of the drug, particularly neutropenia and febrile neutropenia, and showed even more significant benefits than 3-weekly regimen (15,16). So, weekly administration of docetaxel seems to be safe and effective.
Carboplatin and docetaxel perform differently in antitumor and most of their toxicities do not overlap, their combination may yield higher efficacy than either alone. Other studies have observed that docetaxel plus carboplatin had RRs of 39% and 43% and tolerable toxicity (9,17). However controversy still exists regarding which schedule and dosage for the combination of docetaxel and carboplatin results in the optimum clinical outcome while minimizing hematologic and nonhematologic toxicities.
Therefore, we designed this phase II trial to evaluate the efficacy, safety of a weekly docetaxel and carboplatin in NSCLC.
Methods
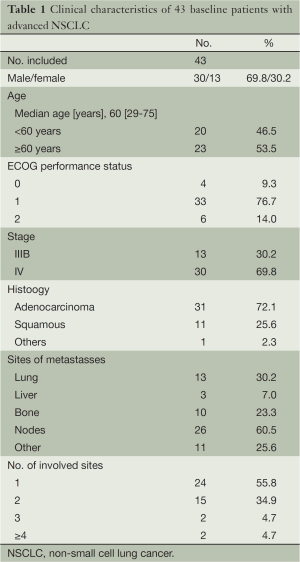
Patients’ eligibility (Table 1)
Full table
The patients enrolled from 18 to 75 years old, were confirmed cytologically or histologically of stage IIIB or stage IV NSCLC, with a measurable tumor examined clinically and/or radiologically. The patients were also required to have an ECOG PS of 0 to 2 and a life expectancy of more than three months. Laboratory requirements included hemoglobin 9 g/dL, white blood cell count 4,000/mm3, neutrophils 2,000/mm3, platelets 100,000/mm3, total bilirubin 1.5 mg/dL, transaminase 1.5 times the institutional upper limit of the normal value, serum creatinine 1.5 mg/dL, and partial pressure of oxygen in artery (PaO2) 60 mmHg. Patients were excluded if they were founded have symptomatic brain metastases, active double cancer, or a severe comorbidity, included symptomatic cardiovascular disease, uncontrolled diabetes, pulmonary fibrosis obvious in a chest X-ray, or a sever infectious disease. The study protocol was approved by the Institutional Review Board at First Affiliated Hospital, College of Medicine, Zhejiang University. Every patient signed a written informed consent before participating in the study
Drug administration and modification
Patients were administered docetaxel intravenously at a dose of 35 mg/m2 on days 1, 8 and 15 with carboplatin at an area under the curve (AUC) 5 on day 1 every 28-day cycle. The Cockroft-Gault equation was used to evaluate the creatinine clearance. It is necessary that patients were injected antiemetic agents and antagonist before they received the anticancer agents.
The National Cancer Institute Common Toxicity Criteria version 3.0 was regarded as the standard to estimate the toxicities during the administration of antitumor agents. If the neutrophil count and the platelet count were more than 1,000/mm3 and 75,000/mm3, respectively, the administration of docetaxel was permitted on days 8 and 15. On the contrary, dose reduction happened to caboplatin to AUC 4 on condition of febrile neutropenia less than 1,000/mm3, thrombocytopenia less than 20,000/mm3, or the demand of platelet transfusion. When a major nonhematological toxicity was at least grade 3, dose reduction was required to eliminate anorexia or nausea. If these kinds of toxicities were continued, the dose of docetaxel was to be decreased in the following cycle.
All patients were required to receive at least two cycles of the treatment, excepting the circumstances of their disease progression, unacceptable toxicities, the patients’ refusal of further treatment or the physicians’ decision of terminating the treatment.
Treatment assessment
patients were asked to accept the following baseline assessment items: a complete medical history and physical examination, complete blood cell counts, hepatic and renal function tests, urinalysis, 12-lead electrocardiograph, and PS. Method of measurement for visible and palpable tumors at baseline included chest X-ray, chest and upper abdominal computed tomography (CT), brain CT or magnetic resonance imaging, a radionuclide bone scan, and other diagnostic procedures were performed as clinically indicated. It is significant to monitor weekly the medical history, physical examination, weight, vital signs, PS, toxicity, complete blood cell counts and blood chemistry. Patients’ response to the treatment was assessed by chest and upper abdominal CT every two cycles and sooner, if required, to document disease progression. According to the new Health Organization criteria (REECIST criteria), the unidirectional items were defined as: complete response (CR)—the disappearance of all lesions, partial response (PR)—a decrease of at least 30% in the sum of the longest diameter of the tumor, progressive disease (PD)—an increase of at least 20% of the longest diameter of the tumor or the appearance of any new lesions, and stable disease (SD)—any response other than CR, PR, or PD. The RR was defined as the proportion of the patients who attained CR or PR to the number of enrolled patients. Overall survival (OS) and time to progression (TTP) were assessed from the start of therapy until death or progression, respectively, or until the last follow-up. Toxicities were estimated in accordance with Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
Study design and statistical analysis of the trial
The primary objective of this study was to evaluate the overall RR, as well as the secondary were TTP, OS, toxicities and safety. The Optimal Simon two-stage phase II design performed as the determination of the sample size and interim analysis was implemented as the first 13 eligible patients had been recruited (11). If more than three responses were observed, 30 patients were to be enrolled, or the study was to be terminated. Withal, when more than 12 responses were to be found in the 43 patients, it was reasonable to regard the regimen as a sufficient and active one with a significance level of 5%. In addition, power of 80% was to be referred for further estimation. The Kaplan-Meier method was considered as the appropriate one to analyze TTP and OS, which were updated to 1 January 2009. On the basis of SPSS (version 10.0), the study undertook a series of statistical computations.
Results
Patient characteristics (Table 1)
Between December 2005 and July 2007, 43 advanced NSCLC Chinese patients were enrolled with 30 males and 13 females. The characteristics of these patients were showed in Table 1. Among them, 76.7% patients had PS 1, and 6 patients had PS 2. Their median age was 60 years (range, 29-75 years) and 21% of the patients were aged over 70. In the respect of histology, adenocarcinoma (72.1%) held a predominant position. Thirteen (30%) patients were at the stage IIIB and other 30 (70%) stage IV.
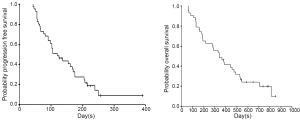
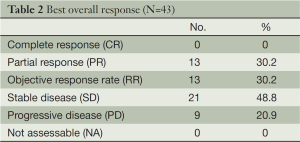
Tumor response and survival (Table 2) (Figure 1)
Full table
In total, 153 chemotherapy cycles were administrated for 43 eligible patients, with a median of 4 cycles per patient (range, 2-6 cycles). Of the 43 eligible patients, the overall RR was 30.2% (95% CI: 17-46%) and the best response to treatment was the PR in 13 patients (30.2%). SD was observed in 21 patients (48.8%) and PD in 9 patients (20.9%).
Survival analysis was implemented at the close-out date (Jan 1, 2009), at which 9 patients (20.9%) were still alive and other 34 patients (79.1%) had died. Thus, the median OS was 340 days (95% CI: 224-456 days). A percent of 46.5 patients survived at one year and 20.0% at two years. Furthermore, as at the close-out date, 2 patients were observed alive without disease progression but 9 patients (20.9%) had progressed. The median progression-free survival time was 120 days (95% CI: 80-160 days), with 9.35% patients (95% CI: 1.76-20.5%) estimated without progression.
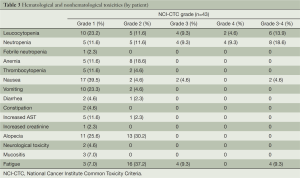
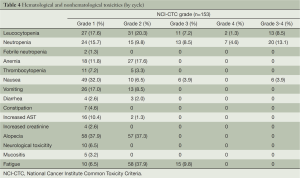
Toxicity (Tables 3,4)
Full table
Full table
There was the evaluation of toxicity for all patients. In total, 16 (37.2%) received 2 cycles, 2 (4.7%) 3 cycles, 15 (34.9%) 4 cycles, 5 (11.6%) 5 cycles and 5 (11.6%) 6 cycles. About the treatment delay, 4 cycles (19%) in 4 patients were more than seven days, of which, 18 cycles for toxicity reasons.
Neutropenia, the most common hematological grade 3/4 adverse event, was observed in 8 patients (18.6%). Leucocytopenia was in 6 patients (13.9%). There was only one (2.3%) patients founded suffering with febrile neutropenia. Additionally, nonhematological toxicities were relatively moderate. All grade of the nonhematological toxicities, nausea, vomiting, alopecia and fatigue held the proportion of 48.8% (grade 3/4 4.6%), 27.9%, 55.8% and 53.5% (grade 3/4 9.3%), respectively. Of the total 153 cycles, grade 3/4 neutropenia occurred in 20 (13.1%) cycles and grade 3/4 leucocytopenia in 13 cycles (8.5%). Grade 3 nonhematological toxicities were observed in 6 cycles (3.9%).
Discussion
In this phase II study, we investigated the activity and safety of weekly docetaxel plus carboplatin in patients with advanced NSCLC. The results showed that the regimen was effective for advanced NSCLC. Of the 43 eligible patients, we observed an overall objective RR of 30.2% (95% CI: 17-46%), the 1-year survival rates of 46.5%, PFS of 120 days (95% CI: 80-160 days), and OS of 340 days (95% CI: 224-456 days), respectively. In previous phase II study, triweekly docetaxel plus carboplatin showed the overall RRs were 42% to 44%, the 1-year survival rates was 50% to 53% and the median OS was 12.0-15.0 months (17,18). In recent phase III study, docetaxel plus carboplatin was observed that the overall RR was 24% and the 1-year survival rate was 38% (19). In another phase II trial, biweekly docetaxel combined with carboplatin showed the overall RR of 30%, the 1-year survival rate of 50%, and the median OS of 11.8 months (9). Furthermore, our data confirmed the previous observations that weekly docetaxel and carboplatin showed the overall RR of 36.7%, the 1-year survival rate of 37.6%, TTP of 5.2 and OS of 10.4 months (20). So, our weekly regimen was comparable with those studies in the aspect of efficacy and the survival results.
As regarding hematological toxicity, our patients experienced the more moderate adverse effects than that of triweekly docetaxel plus carboplatin in which 70% of patients were observed with neutropenia, and 15% with febrile neutropenia (9,11,17,19). Comparatively, in our weekly regimen, the grade 3 or 4 neutropenia was observed in eight (18.6%) patients and there was only one (2.3%) patients suffered with febrile neutropenia, which suggested that our weekly regimen might be safer. So, weekly docetaxel was more advantageous in hematological toxicity than that of 3-weekly schedule (21,22).
For nonhematological toxicities, they were mild and moderate to be managed. Grade 3 or 4 nonhematological toxicities were rare in this study (4.6% nausea, and 9.3% fatigue). Contrasted to previous findings, severe diarrhea or pulmonary toxicity was barely observed in the administration of our weekly regimen (9,23).
The use of weekly docetaxel, rather than traditional 3-weekly, has been investigated in elderly patients, for reducing toxicity of myelosuppression (24). In our study, the elderly population holds a proportion of 53.5%, and there is no difference in the patient characteristics, such as PS or stage between younger patients and elderly patients. However, our study was not terminated because of these more than half of the elderly patients. Moreover, weekly docetaxel with carboplatin illustrated significant efficacy. So, weekly docetaxel and carboplatin might also be a good choice for elderly patients; however it needed further large, randomized studies to evaluate this result.
In conclusion, the combination of weekly docetaxel and carboplatin showed feasible efficacy with acceptable hematologic toxicities for advanced NSCLC.
Acknowledgements
This study was sponsored by Zhejiang Provincial Science and Technology Project (Grant No. 2011C23087), Zhejiang Provincial Traditional Chinese Medicine Science Research Foundation (Grant No. 2012ZA078).
Disclosure: The authors declare no conflict of interest.
References
- Ferlay J, Bray F, Pisani P, et al. GLOBOCAN 2000: cancer Incidence, mortality and prevalence worldwide, Version 1.0. IARC CancerBase No. 5. Lyon, IARC Press, 2001.
- Parkin DM, Bray FI, Devessa SS. Cancer burden for the year 2000: the global picture. Eur J Cancer 2001;37 Suppl 8:S4-66. [PubMed]
- Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med 2004;350:379-92. [PubMed]
- Higgins MJ, Ettinger DS. Chemotherapy for lung cancer: the state of the art in 2009. Expert Rev Anticancer Ther 2009;9:1365-78. [PubMed]
- Raez LE, Lilenbaum R. New developments in chemotherapy for advanced non-small cell lung cancer. Curr Opin Oncol 2006;18:156-61. [PubMed]
- Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000;18:2095-103. [PubMed]
- Spigel DR, Greco FA. Chemotherapy in metastatic and locally advanced non-small cell lung cancer. Semin Surg Oncol 2003;21:98-110. [PubMed]
- Barlési F, Jacot W, Astoul P, et al. Second-line treatment for advanced non-small-cell lung cancer: a systematic review. Lung Cancer 2006;51:159-72. [PubMed]
- Cho SH, Go SI, Lee GW, et al. Phase II study of a biweekly schedule of docetaxel and cisplatin in patients with metastatic non-small cell lung cancer. Lung Cancer 2010;69:94-8. [PubMed]
- Dancey J, Shepherd FA, Gralla RJ, et al. Quality of life assessment of second-line docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy: Results of a prospective, randomized phase III trial. Lung Cancer 2004;43:183-94. [PubMed]
- Hotta K, Matsuo K, Ueoka H, et al. Meta-analysis of randomized clinical trials comparing Cisplatin to Carboplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol 2004;22:3852-9. [PubMed]
- Ardizzoni A, Boni L, Tiseo M, et al. Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data meta-analysis. J Natl Cancer Inst 2007;99:847-57. [PubMed]
- Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000;18:2095-103. [PubMed]
- Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small- cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small-Cell Lung Cancer Study Group. J Clin Oncol 2000;18:2354-62. [PubMed]
- Schuette W, Nagel S, Blankenburg T, et al. Phase III study of second-line chemotherapy for advanced non-small-cell lung cancer with weekly compared with 3-weekly docetaxel. J Clin Oncol 2005;23:8389-95. [PubMed]
- Lilenbaum RC, Schwartz MA, Seigel L, et al. Phase II trial of weekly docetaxel in second-line therapy for non-small cell lung carcinoma. Cancer 2001;92:2158-63. [PubMed]
- Belani CP, Einzig A, Bonomi P, et al. Multicenter phase II trial of docetaxel and carboplatin in patients with stage IIIB and IV non-small cell lung cancer. Ann Oncol 2000;11:673-8. [PubMed]
- Zarogoulidis K, Kontakiotis T, Hatziapostolou P, et al. A Phase II study of docetaxel and carboplatin in the treatment of non-small cell lung cancer. Lung Cancer 2001;32:281-7. [PubMed]
- Fossella F, Pereira JR, von Pawel J, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol 2003;21:3016-24. [PubMed]
- Jiang L, Wang D, Zhu Z, et al. Phase II study of carboplatin combined with weekly docetaxel in patients with advanced non-small cell lung cancer. Cancer Chemother Pharmacol 2010;66:449-53. [PubMed]
- Gridelli C, Gallo C, Di Maio M, et al. A randomized clinical trial of two docetaxel regimens (weekly vs 3-week) in the second line treatment of non-small cell lung cancer. The DISTAL 01 study. Br J Cancer 2004;91:1996-2004. [PubMed]
- Camps C, Massuti B, Jiménez A, et al. Randomized phase III study of 3-weekly vs weekly docetaxel in pretreated advanced non-small-cell lung cancer: a Spanish Lung Cancer Group trial. Ann Oncol 2006;17:467-72. [PubMed]
- Esteban E, González de Sande L, Fernández Y, et al. Prospective randomised phase II study of docetaxel versus paclitaxel administered weekly in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. Ann Oncol 2003;14:1640-7. [PubMed]
- Hainsworth JD, Burris HA 3rd, Litchy S, et al. Weekly docetaxel in the treatment of elderly patients with advanced nonsmall cell lung carcinoma. A Minnie Pearl Cancer Research Network Phase II Trial. Cancer 2000;89:328-33. [PubMed]