Is video-assisted thoracic surgery a versatile treatment for both simple and complex pulmonary aspergilloma?
Introduction
Decision to preform surgical resection has been controversial since pulmonary aspergilloma (PA) was classified into simple pulmonary aspergilloma (SPA) and complex pulmonary aspergilloma (CPA) by Belcher and Pulmmer (1). Generally, surgical treatment for SPA is acceptable because the surgical risk is minimal. In contrast, resection is limited to low-risk patients in CPA because surgery for CPA patients had been reported with more complication and higher mortality (2-4). Over the past few years, video-assisted thoracic surgery (VATS) has been increasingly applied for both benign and malignant pulmonary disease because of its optimal results such as less morbidity and rapid recovery. Although Qian-Kun Chen had reported that VATS was feasible for PA (5), the risk of massive hemorrhage and high level of complication still exist in CPA patients treated by VATS, further studies are still necessary before VATS can be widely accepted as a standard treatment for PA patients, particularly the CPA patients. Here, we performed VATS for 16 patients with PA, surgical outcomes of both SPA and CPA were compared in this study.
Patients and methods
Between October 2009 and March 2013, 16 patients were consecutively treated by VATS for PA in our department. Record of each patient was retrospectively reviewed regarding the preoperative, perioperative and postoperative information. This study was approved by the first affiliated hospital of Zhejiang University medical school review board of clinical research. The need for informed consent from patients was waived because of the retrospective design of the study.
Diagnosis of aspergilloma was made on the basis of medical history, clinical symptoms and computed tomography (CT)-scan image. Sputum cultures were not routinely performed preoperatively. Fiberoptic bronchoscopy was not recommended to perform because it may cause server cough and aggravate hemoptysis. All specimens from resected lung had pathologic confirmation of PA.
The types of aspergilloma were classified retrospectively based on CT-scan image findings and the report of Belcher and Plumme (1): aspergilloma is considered as SPA when it develops in isolated thin-walled cysts (≤3 mm) lined by ciliated epithelium with little or no surrounding parenchymal disease. In contrast, CPA has a thick-walled (>3 mm) cyst with surrounding parenchymal disease such as tuberculosis, bronchiectasis, chronic lung abscess and greater pleural thickening. Our criteria for the selection of patients who received the VATS is based on the following two conditions: (I) young patients (≤65 years) without severe pulmonary parenchyma disease; (II) there is no severe pleural thickness which may suggest obvious pleural adhesions. In addition, patients with invasive aspergilloma and calcified lymph nodes near pulmonary arteries and veins were excluded.
All surgeries were performed by the same experienced surgeon in our department. Patients were placed in lateral decubitus and all procedures were conducted under general anesthesia with single-lung ventilation. Exploratory VATS using two lower thoracoscopic ports was performed, one 7 mm and two 1 cm access incisions were made on the lesion side at the fourth intercostal space on the anterior axillary line, the seventh intercostal space on the midaxillary line and the eighth or ninth intercostal space on the posterior axillary line. In general, lesion and all major pulmonary vessels and bronchi in the affected lobe were resected using endoscopic staplers. One of the incisions was extended approximately to 2.0-3.5 cm in length to facilitate the removal of the resected lobe from the thoracic cavity with a retrieval bag. One or two chest tubes were placed at the end of the procedure depending on hemorrhage level of each patient, the tubes were removed if there is no air leak and the daily output was less than 200 mL. Patients showed no main complication or no obvious pneumothorax and chest fluid by X-rays were discharged from the hospital. In our series, antifungal agents were not used preoperatively but all patients were told to take antifungal agent (Fluconazole) for two weeks with the dosage of 500 mg per day when they are discharged from hospital.
Follow-up data were obtained from the outpatient clinic chart reviews and telephone call to patients or families. The follow-up process ended in October 2013. Data were analyzed using SPSS version 20.0 (SPSS Inc. Chicago, III, USA). The association between variables was analyzed by either the chi-square or t-test. Statistical significance was defined as P value is less than 0.05. Categorical variables were analyzed using Fisher exact test.
Results
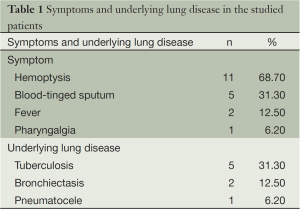
The patients included ten men and six women with a median age of 52.8 years (95% CI: 47.8-57.9 years). Among the 16 patients, one patient had systematic underlying diseases of hypertension and diabetes. The most common symptom was hemoptysis (≥100 mL, 68.7%) followed by blood-tinged sputum (Table 1: tuberculosis (five cases, 31%) represented the main underlying disease in CPA patients, the rest were bronchiectasis (two cases, 13%) and pneumatocele (one case, 6%).
Full table
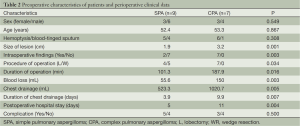
Comparison of the baseline demographic characteristics and perioperative clinical findings between patients with SPA and CPA are shown in Table 2. Five patients (55.6%) in SPA group and six patients (85.7%) in CPA group presented the symptom of hemoptysis. Operations were performed electively. Intra-operative finding included varying degrees of pleural adhesions and pleural wall thickening, pleural adhesion was observed in six patients (85.7%) and pleural thickening was found in two patients (28.6%). No patients was converted to thoracotomy, all lesion were resected successfully.
Full table
There was no difference in terms of gender, age and occurrence of hemoptysis. However, the patients in CPA group had larger size of lesion (mean =3.2 cm, 95% CI: 2.7-3.7 cm, P=0.001) and intra-operative findings (P=0.003) (Table 2) compared with those in the SPA group. In addition, data indicated that CPA patients were more likely to be selected to conduct lobectomy and operation time was relatively longer (mean =187.9 min, 95% CI: 158.2-217.5 min, P=0.016) with more blood loss (mean =150 mL, 95% CI: 72.8-227.2 mL, P=0.003) (Table 2). The amount of chest drainage was significantly less in SPA group (mean =523.3 mL, 95% CI: 386.6-660.1 mL, P=0.005) coupled with relatively shorter duration of chest drainage (mean =3.9 days, 95% CI: 2.8-4.9 days, P=0.007) and postoperative hospital stay (mean =5 days, 95% CI: 4.0-6.0 days, P=0.004) (Table 2).
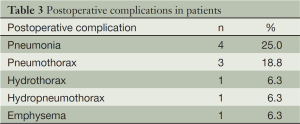
There was no perioperative deaths in either group, ten complications developed in eight patients (50%): four pneumonia, three pneumothorax, one hydrothorax, one hydropneumothorax, and one emphysema (Table 3), no significance difference was found in the occurrence of complications. The mean follow-up period was 21.6 months (95% CI: 16.1-26.7 months). One patient was lost to follow-up in SPA group because of the changed telephone number. Satisfyingly, further complications, recurrence and PA-related death were not found among the other patients.
Full table
Discussion
PA generates from inhalation of aspergillus-reproduced conidia through airways. These aspergillus organisms mainly colonize in pre-existing pulmonary cavities of aspergillus fumigatus. CPA usually develops in benign cavitary pulmonary disease such as tuberculosis, bronchiectasis and lung abscess. The most common cavitary lesion in all series is tuberculosis (3,6,7), consistent with our experience: tuberculosis was the main cause of cavitary lung lesions in 31% of our cases. It is reported that the most common symptom associated with aspergilloma is hemoptysis (8). Consistently, our data demonstrated that hemoptysis (≥100 mL) serves as the leading symptoms (68.7%) followed by blood-tinged sputum (Table 1). No significance difference was found between SPA and CPA group in number of patients who developed hemoptysis and blood-tinged sputum, and there was no occurrence of emergency hemoptysis attack in CPA group, indicating that hemoptysis cannot be considered as a characteristic feature to distinguish CPA from SPA in our study.
Lesion excision is considered as the mainstay treatment of PA, it not only reduce the incidence of life-threating hemoptysis but also result in a likelihood of a permanent cure (3,9,10). The decision of surgery procedure of VATS depends on the balance between the risk of life-threating hemorrhage in surgery and benefit of rapid postoperative recovery. Wedge resections under VATS have been widely used for patients with benign and malignant lung disease due to its safety, minimal time consumption and reliability. It only requires 2-3 small, 1-1.5 cm long incisions. Whereas lobectomy, another procedure which causes more injury, is inevitable for the limitation of lesion site and size in some cases. In our practice, we take wedge resection as priority in case of SPA with peripheral and smaller lesion, because the surgical risk is minimal. Shirakusa wrote that wedge resection can be performed in patients when aspergilloma is sufficient small and located in the healthy lung periphery (11). However, we must evaluate the patients and only recommended wedge resection for low-risk patients without infiltration of the hilum, no matter SPA or CPA patients, because dissection of lesion and calcified lymph node near pulmonary arteries is of enormous jeopardy. Based on that, four patients in SPA group were performed wedge resection but none of CPA patients was candidate for wedge resection.
PA lesions are more difficult to be surgically dissected in patients with CPA. The dense fibrosis and diseased pulmonary surrounded the enlarged cavity made resection of PA lesion via VATS more technically challenging. Besides, obliteration of pleural space and fissures increased the risk of surgical bleeding. Adhesions accompanied with more proliferative vessels made the surgical field bloody and blurred during dissection, although the interchangeable use of the electrocoagulation hook and ultrasonic dissector effectively kept the surgical field clean, the separation of pleural adhesions definitely accounted for longer time consuming and more blood loss, and prolonged postoperative chest drainage and hospital stay.As for postoperative complications, no significant differences were found between the two groups. In SPA group, three patients had pneumonia, and they were managed by antibiotics. One patient had pneumothorax and one patient had hydrothorax, they were managed by prolonged chest tube drainage. In CPA group, two patients had pneumothorax and one patient had hydropneumothorax combined with emphysema, this patient is a 49 aged male with tuberculosis and recurrent pneumothorax. This woman was managed by conservative comprehensive treatment.Complications such as wound infection and empyema is caused mainly by contamination of pleural cavity and wound during resection of infected or purulent lesions. Some researcher wrote that the routine usage of neomycin sulfate solution before closing would effectively avoid the intraoperative dissemination (5). In our institute, we use a mass of 0.5% povidone-iodine to clean the cavity and incision to avoid contamination, usage of antibiotic rinse is not recommended because of the possible generation of antibiotic resistance, which makes the treatment of unpredictable infection or recurrence of PA thornier.None of our patients experienced bronchopleural fistula, which was considered as one of the most serve complications and happened in 1.6% to 15.8% of PA patients in previous studies treated by surgeries (12-14). Our favorable outcomes probably attributed to the usage of intercostal muscle, pleural flaps, or a pericardial fat pad to cover the bronchial stump. Moreover, avoiding of excessive dissection is highly recommended to preserve perfusion for healing of the bronchial stump.Good underlying pulmonary condition and postoperative care are essential for preventing postoperative mortality, because most deaths are caused by chronic respiratory failure or pneumonia (15). In our study, mortality was not found in either group during follow-up period. Previous studies reported high mortality ranging from 1.4% to 34.3% of patients with CPA treated by thoracotomy (2). In the literate reports about VATS for PA, only one CPA patient died after operation, and that patient is a 70-year-old male with a history of surgeries for esophageal cancer (2,5). The minimal invasive approach for lung resection and the early postoperative recovery of VATS are considered to attribute to the favorable mortality results. The different severity of underlying disease and a more stringent patient selection in this study may explain the improved results as well, we must declare that our patients were relatively young and there was no existing of serious systematic underlying disease or local pulmonary disease such as lung and esophageal cancer in our patients.There are several limitations to this study because of its retrospective design. The first limitation of this study concerns the relatively small cases volume. More patients in China will choose thoracotomy because of the higher expense of VATS, and some records of patients are lost during the transformation of the medical record system in our hospital. The second limitation lies in the long time span of this study, the treatment effect might be different with the increased experience of surgeon performing VATS as time goes on.
Conclusions
SPA patients with small and peripheral lesion are best candidates for wedge resection by VATS. Although the mortality of CPA patients treated by VATS is not inferior to thoracotomy, comprehensive measure should be taken for the overall benefit of CPA patients before conducting VATS. Application of VATS should not be pursued as something trendy, accumulation of the further cases are necessary before VATS can be widely accepted as a standard treatment for CPA patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Belcher JR, Plummer NS. Surgery in broncho-pulmonary aspergillosis. Brit J Dis Chest 1960;54:335-41.
- Ichinose J, Kohno T, Fujimori S. Video-assisted thoracic surgery for pulmonary aspergilloma. Interact Cardiovasc Thorac Surg 2010;10:927-30. [PubMed]
- Kim YT, Kang MC, Sung SW, et al. Good long-term outcomes after surgical treatment of simple and complex pulmonary aspergilloma. Ann Thorac Surg 2005;79:294-8. [PubMed]
- Daly RC, Pairolero PC, Piehler JM, et al. Pulmonary aspergilloma. Results of surgical treatment. J Thorac Cardiovasc Surg 1986;92:981-8. [PubMed]
- Chen QK, Chen C, Chen XF, et al. Video-assisted thoracic surgery for pulmonary aspergilloma: a safe and effective procedure. Ann Thorac Surg 2014;97:218-23. [PubMed]
- Akbari JG, Varma PK, Neema PK, et al. Clinical profile and surgical outcome for pulmonary aspergilloma: a single center experience. Ann Thorac Surg 2005;80:1067-72. [PubMed]
- Csekeo A, Agócs L, Egerváry M, et al. Surgery for pulmonary aspergillosis1. Eur J Cardiothorac Surg 1997;12:876-9. [PubMed]
- Hemphill RA. Mycotic lung infection. Am J Med 1946;1:708. [PubMed]
- Battaglini JW, Murray GF, Keagy BA, et al. Surgical management of symptomatic pulmonary aspergilloma. Ann Thorac Surg 1985;39:512-6. [PubMed]
- Jewkes J, Kay PH, Paneth M, et al. Pulmonary aspergilloma: analysis of prognosis in relation to haemoptysis and survey of treatment. Thorax 1983;38:572-8. [PubMed]
- Shirakusa T, Ueda H, Saito T, et al. Surgical treatment of pulmonary aspergilloma and aspergillus empyema. Ann Thorac Surg 1989;48:779-82. [PubMed]
- Chen QK, Jiang GN, Ding JA. Surgical treatment for pulmonary aspergilloma: a 35-year experience in the Chinese population. Interact Cardiovasc Thorac Surg 2012;15:77-80. [PubMed]
- Brik A, Salem AM, Kamal AR, et al. Surgical outcome of pulmonary aspergilloma. Eur J Cardiothorac Surg 2008;34:882-5. [PubMed]
- Caidi M, Kabiri H, Al Aziz S, et al. Surgical treatment of pulmonary aspergilloma. 278 cases. Presse Med 2006;35:1819-24. [PubMed]
- Moreau P, Zahar JR, Milpied N, et al. Localized invasive pulmonary aspergillosis in patients with neutropenia. Cancer 1993;72:3223-6. [PubMed]


