Lentivirus vector-mediated Rho guanine nucleotide dissociation inhibitor 2 induces beta-2 adrenergic receptor desensitization in β2AR desensitization mice model
Introduction
Airway smooth muscle can be relaxed by β-adrenoceptor stimulation. This primarily involves β2-adrenoceptors in human airways while airway β2-adrenoceptors are sensitive to agonist-induced desensitization. The initial β-adrenoceptor response to salbutamol treatment is active; however, the treatment efficacy decreases with the duration and therapeutical cycles increasing. Desensitization is acquired rapidly, contributing to therapy failure. However, the mechanisms by which β-adrenoceptor develop desensitization to salbutamol are not fully understood. Previous study showed that Rho guanine nucleotide dissociation inhibitor 2 (RhoGDI2) level increased in asthmatic murine model of β2-adrenoceptor desensitization by a proteomics approach (1). While the association and function of RhoGDI2 with β2AR in β2AR desensitization remains unclear.
RhoGDI2 has been identified as a regulator of RhoGTPase (2). It is mainly in hematopoietic, endothelial, and epithelial cells (3,4). RhoGDI2 has been linked to tumorigenesis and metastasis. Its precise role in cancer varies with tumor type (5). RhoGDI2 expression is downregulated in several cancer types, such as bladder, lung and lymphoma (6,7), but is upregulated in prostate and gastric cancer (8,9). Further, RhoGDI2 protein was significantly upregulated in high-grade compared with low-grade ovarian cancers, correlated with histological subtype, and did not correlate with stage of ovarian cancer (10). Thus, RhoGDI2 appear to carry out different functions within the same tumor type.
Previous study had shown that RhoGDI2 was overexpressed in chemotherapy-resistant paclitaxel-resistant ovarian cancers and fibrosarcoma cells, respectively (11). At the same time, it was reported that RhoGDI2 conferred resistance against cisplatin-induced apoptosis in gastric cancer cells (12). In previous study revealed that RhoGDI2 is a contributor to 5-FU resistance in colon cancer (13). The subsequent study also demonstrated that RhoGDI2 also confers resistance to 5-FU in gastric cancer cells (14). The results lead to the conclusion that high levels of RhoGDI2 expression are associated with chemotherapy resistance in certain types of cancers. Our previous study showed that lentivirus vector-mediated RhoGDI2 overexpression induces beta-2 adrenergic receptor (β2AR) desensitization in airway smooth muscle cells. This study was to further study RhoGDI2 induces β2AR desensitization in mice model.
To date, there has been no report on the significance of RhoGDI2 expression for β2AR desensitization. Hence, in this study, we studied the effects of RhoGDI2 on β2AR desensitization and the underlying mechanism in vivo.
Materials and methods
Reagents
Lentivirus vectors were created as previously described (15-17). The RhoGDI2 gene (NCBI NM_001175.4) coding sequence was amplified by PCR and subcloned into a lentivirus expression plasmid pWPXL-eGFP vector (TronoLab, France) along with BamH I and Mlu I restriction sites to construct a lentivirus-based overexpression vector carrying the RhoGDI2 sequence (pWPXL-eGFP-RhoGDI2), confirmed by PCR and DNA sequencing. Additionally, siRNA interference sequences of RhoGDI2 were designed based on GenBank. Interference sequences were synthesized by Biomics Biotechnologies Co., Ltd (China). Lentivirus expression plasmids were cotransfected into 293T cells to construct pLenti-GDI viral stock and pRNAi-GDI viral stock. Lentivirus vector stocks with titers ranging from 0.1×109 to 2.0×109 particles/mL were used.
Mice and in vivo delivery of lentivirus supernatant
For experiments BALB/c mice were used at 6-8 weeks of age. All animal care and surgical procedures were carried out in accordance with the Guide for Care and Use of Laboratory Animals (National Research Council, 1996, USA) and were approved by the Chinese National Committee to Use of Experimental Animals for Medical Purposes, Jiangsu Branch. All efforts were made to minimize the number of animals used and their suffering. Lentiviral vectors were delivered intratracheally (i.t.) as previously described (18,19). In brief, the neck was extended and cleaned with a chlorhexidine solution. A small midline incision was made with a sterile scalpel to expose the trachea. About 0.5×108 to 1×108 vector particles in a final volume of 50 μL of phosphate-buffered saline (PBS) were instilled into the trachea with a 27-gauge needle. RhoGDI2 overexpression group (n=10)—intratracheal delivery of pLenti-GDI Viral Stock; RhoGDI2 siRNA group (n=10)—intratracheal delivery of pRNAi-GDI Viral Stock; empty viral vector group (n=10)—intratracheal delivery of the empty viral stock; experimental control group (n=10) and blank control group (n=5)—intratracheal delivery of the same volume of PBS. Upon completion of the instillation, the skin and fascia were closed in one layer with interrupted sutures (4-0 Vicryl). RhoGDI2 overexpression group, RhoGDI2 siRNA group, empty viral vector group, experimental control group underwent the same procedure daily intraperitoneal injection of 60 µg salbutamol and inhaling an aerosol of 0.01% salbutamol 30 min/d the same day as intratracheal delivery of lentiviral vectors for 21 days. But blank control group was treated with PBS. Mice were allowed freely to get water and food and housed under a 12-hour light-dark cycle. Room temperature was kept at 22±0.5 °C.
Measurement of airway hyperreactivity
At the 21 days, the mice were anesthetized by intraperitoneal injection of 1% pentobarbital sodium 0.15 mL. Hyperresponsiveness to increasing concentrations of acetylcholine chloride (Ach) was measured through a whole-body plethysmograph system (AniRes2005, Beijing SYNOL High-Tech Co. Ltd., China) and by determining airway expiration resistance (Re).
RNA extraction and reverse transcription-PCR
Mice were killed after measuring airway hyperreactivity. Fresh lung tissue samples were obtained and stored at –80 °C until extraction. Total RNA was extracted using Trizol® reagent (Invitrogen™ life technologies). After complete treatment of RNA with RNase-Free DNase (Promega) for 45 min at 37 °C, a cDNA library was generated using M-MLV reverse transcriptase (Promega) and oligo (dT) primers. For PCR amplification, specific oligonucleotide primer pairs (10 pmol each) were incubated with 1 μL of cDNA template in a 20 μL PCR reaction mixture. Primer sequences were: 5'-GCCTGAGGAGTATGAGTTC-3'(F) and 5'-GAGGTGGTCTTGCTTGTC-3'(R) for RhoGDI2; 5'-AGATCAAGAAGTACGAGAAG-3' (F) and 5'-GATGTATGGCTGGAAGAG-3' (R) for GRK2; 5'-CCTGCTGACCAAGAATAAG-3' (F) and 5'-AAGGACACGATGGAAGAG-3' (R) for β2AR; 5'-CCATTTGCAGTGGCAAAG-3' (F) and 5'-CACCCCATTTGATGTTAGTG-3' (R) for GAPDH. Dilutions of the cDNAs were amplified for 35 cycles at 94 °C for 30 sec, 60 °C for 30 sec, and 72 °C for 30 sec. The amplified PCR products were analyzed by 1% agarose gel electrophoresis and gel red staining.
Western blot
The fresh lung tissue samples were homogenized in a lysate buffer (5 mmol/L EDTA, 50 mmol/L Tris, 1% SDS, pH 7.5, 10 μg/mL aprotinin, 1% sodium deoxycholate, 1% NP-40, 1 mM PMSF, 1% Triton-X 100, and 10 μg/mL leupeptin) and then centrifuged in a microcentrifuge at 4 °C for 20 min to collect the supernatant. Protein concentrations were determined with the Bradford assay (Bio-Rad). After diluted with SDS loading buffer and boiled protein samples were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to polyvinylidine difluoride filter (PVDF) membranes (Millipore). The membranes were blocked with 5% dried skim milk in TBST (20 mM Tris, 150 mM NaCl, 0.05% Tween-20). After two hours at room temperature, the membranes were washed and incubated with primary antibody against RhoGDI2 (anti-rabbit, 1:500; Epitomics), GRK2 (anti-rabbit, 1:500; Epitomics), β2AR (anti-rabbit, 1:100; Santa Cruz), Glyceraldehyde-3-phosphate Dehydrogenase (GAPDH, anti-rabbit, 1:1,000; Sigma) at 4 °C overnight. After incubating with horseradish peroxidase-conjugated secondary antibody, the protein was visualized using ECL (Pierce Company, USA).
Measurement of GRK activity
GRK enzymatic activity was assessed using light-dependent phosphorylation of rhodopsin (Benovic et al., 1987). Rod outer segment (ROS) membranes were prepared from dark-adapted bovine retinas via stepwise sucrose gradient centrifugation, and then treated with 5 M urea to inactivate endogenous kinase activity as substrate. GRK-dependent phosphorylation was determined by incubating 60 μg of lung tissue protein with 0.5 μM ROS in a buffer containing Tris-HCl (pH 7.4) 20 mM, EDTA 2 mM, MgCl2 5 mM, ~1 μCi [ γ- 32P]ATP and 2 nmol ATP in a final reaction volume of 20 μL. The reactions were carried out at 30 °C for 30 min in the presence or absence of light. The incubations were terminated by the addition of 10 μL of 3× sodium dodecyl sulphate (SDS) sample buffer (8% SDS, pH 6.8, 20% glycerol, 50 mM Tris-HCl, 0.005% bromophenol blue and 5% β-mercaptoethanol). Samples were then electrophoresed on 10% PAGE. After electrophoresis, the gel was stained with Coomassie blue, dried, and phosphorylated rhodopsin was visualized by autoradiography. Bands corresponding to rhodopsin (~38 kDa) were cut from the gel and quantitated by liquid scintillation counting.
Statistical analysis
Statistical analyses were performed using SPSS software version 16.0. Each experiment consisted of at least three replicates per condition. All values are expressed as mean ± SEM. Data with a normal distribution were analyzed using a t-test. A P-value of less than 0.05 was considered to be significant.
Results
Airway responsiveness after inhaling Ach
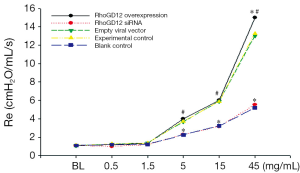
To assess β2AR functional changes, we measured airway hyperreactivity (acetylcholine chloride-induced resistance). β2AR agonists might decrease airway responsiveness, while Ach might trigger airway hyperreactivity. Airway responsiveness determined by airway resistance and described through an animal ventilator after inhaling acetylcholine chloride. Airway responsiveness after inhaling Ach was markedly increased in the RhoGDI2 overexpression group compared to experimental control group and blank control group when concentrations of Ach was 45 mg/mL (all PFigure 1).
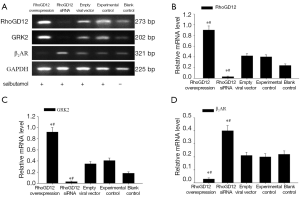
RT-PCR detection of RhoGDI2, β2AR, and GRK2 mRNA expressions
Total RNAs of fresh lung tissue were extracted and mRNA expression of RhoGDI2, GRK2, and β2AR was measured by semi-quantitative RT-PCR. A representative gel of RT-PCR products from the recipient is shown in Figure 2A. The RhoGDI2 and GRK2 mRNA level were significantly increased in RhoGDI2 overexpression group compared to experimental control group and blank control group (all PFigure 2B,C). The RhoGDI2 and GRK2 mRNA level were significantly decreased in the RhoGDI2 siRNA group compared to experimental control group and blank control group (all PFigure 2A-C). The mRNA expressions GRK2 were significantly increased along with increasing RhoGDI2 expression, demonstrating a positive relationship. The mRNA expression of β2AR was significantly lower in the RhoGDI2 overexpression group compared to experimental control group and blank control group (all PFigure 2D).
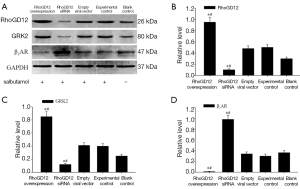
Protein expressions of RhoGDI2, β2AR and GRK2 were detected by western blot
Lung tissues protein from RhoGDI2 overexpression, RhoGDI2 siRNA group, empty viral vector group, experimental control group and blank control group were separated by SDS-PAGE and analyzed by western blotting. As expected, RhoGDI2 and GRK2 protein level were significantly increased in the RhoGDI2 overexpression group compared to experimental control group and blank control group (all PFigure 3A-C). The RhoGDI2 and GRK2 protein level were significantly decreased in the RhoGDI2 siRNA group compared to experimental control group and blank control group (all PFigure 3A-C). Conversely, β2AR expression were significantly lower in the RhoGDI2 overexpression group compared to experimental control group and blank control group (all PFigure 3A,D), exhibiting an inverse correlation with RhoGDI2 expression.
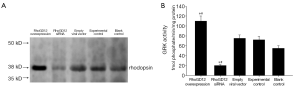
GRK activity in lung
In lung tissue protein from each group, as expected, the GRK enzymatic activity was significantly increased in the RhoGDI2 overexpression group compared to experimental control group and blank control group (all PFigure 4A,B). The GRK enzymatic activity was significantly decreased in the RhoGDI2 siRNA group compared to experimental control group and blank control group (all PFigure 4A,B).
Discussion
β2AR agonists are effective drugs for clinical treatment of respiratory diseases. However, their application is limited because of β2AR desensitization (20). β2AR downregulation is an important aspect of β2AR desensitization (21). In addition, the functional changes of β2AR desensitization were airway responsiveness to cholinergic stimulants.
To study the molecular mechanisms of β2AR desensitization, models should adopt a stimulation method similar to the clinical setting during establishment (22). Therefore, construction of an animal model of β2AR desensitization by stimulating the airway with salbutamol may reflect the molecular mechanisms of β2AR desensitization. Salbutamol is one of the most commonly used short-acting β2AR agonist because of a rapid effect of relieving acute dyspnea, minor systemic side effects, and low-cost (23). Our study adopted salbutamol as the stimulus to construct the animal model, so that it was more similar to the pathological status of clinical patients who are medicated with salbutamol. We constructed the animal model of β2AR desensitization by adding salbutamol over a long-term. The time between drug administration and bronchodilation is various in vivo, it was significantly depended on the β2-adrenoceptor agonist used (e.g., 24).
Our study showed that overexpression of RhoGDI2 had a significant correlation with β2AR desensitization as compared with RhoGDI2 silenced group. However, whether RhoGDI2 may be involved in the development of desensitization to β2AR agonists is not known yet. In this study, we demonstrated that RhoGDI2 induced β2AR desensitization in a murine model by measuring the activity of GRK, protein levels of GRK2, and the total amount of β2AR receptor (western blotting analysis). In addition, the functional changes of airway responsiveness in desensitized mice were measured by airway hyperresponsiveness to Ach. To investigate the mechanisms involved, we used lentivirus-mediated overexpression and siRNA interference targeting RhoGDI2. Forced expression of RhoGDI2 increased tolerance of β2AR to β2AR agonists. Conversely, silence of RhoGDI2 sensitized β2AR to β2AR agonists. These results suggested that silence of RhoGDI2 confers a survival mechanism to β2AR, which protects them from desensitizing these β2AR agonists.
Our date showed that GRK2, an important G protein-coupled receptor kinase that phosphorylates β2ARs specifically targets agonist-occupied receptors (25), expression is upregulated in RhoGDI2-over-expressing salbutamol treated animal model and downregulated in RhoGDI2-depleted salbutamol treated animal model. These results suggested that GRK2 might take part in β2AR agonist tolerance in RhoGDI2-overexpressing salbutamol treated animal model. There are many reports demonstrated the importance of GRK2 in β2AR agonist tolerance and the importance of GRK2 in β2AR agonist tolerance makes GRK2 an important target for the prevention of the development of desensitization (26). Although, how RhoGDI2 upregulates GRK2 expression and whether upregulated GRK2 could mediate resistance to other therapeutic drugs-induced β2AR desensitization in RhoGDI2-expressing animal requires further analyses, all of our results suggested an important role of RhoGDI2 in regulating β2AR desensitization by reinforcing the activity of GRK2 was accompanied with downregulation of β2 adrenergic receptors.
In this study, we demonstrated that RhoGDI2 induced β2AR desensitization. Investigations into the etiology of β2AR desensitization have largely focused on the roles played by GPRKs in mediating phosphorylation of β2AR. Such phosphorylation ultimately results in uncoupling of the agonist-occupied form of the receptor from the G proteins. Although changes in receptor responsiveness may serve as the primary means of desensitization, previous studies demonstrated that homologous desensitization was associated with, but not dependent on, a ~45% loss of cell surface β2ARs (27). These data provide strong evidence that RhoGDI2 regulates β2AR desensitization by indirectly affecting β2AR number and function and may offer a distinct idea from the traditional mechanisms.
In conclusion, our present studies found that RhoGDI2 might induce β2AR desensitization and GRK2 might take part in RhoGDI2-mediated β2AR desensitization in β2AR desensitization mice model. A better understanding the function of RhoGDI2 in β2AR desensitization might provide a new perspective for the prevention and treatment of β2AR desensitization.
Acknowledgements
This work was supported by Natural Science Foundation of China [30971306]; and Six big talent peak in Jiangsu Province project [the seventh batch, 033]; and Nantong social development project [NO: S2009023]; and Nantong fourth period “226 high-level personnel training project” project.
Disclosure: The authors declare no conflict of interest.
References
- Liu H, Zhou LF, Zhang Q, et al. Increased RhoGDI2 and peroxiredoxin 5 levels in asthmatic murine model of beta2-adrenoceptor desensitization: a proteomics approach. Chin Med J (Engl) 2008;121:355-62. [PubMed]
- DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol 2005;15:356-63. [PubMed]
- Theodorescu D, Sapinoso LM, Conaway MR, et al. Reduced expression of metastasis suppressor RhoGDI2 is associated with decreased survival for patients with bladder cancer. Clin Cancer Res 2004;10:3800-6. [PubMed]
- Alcántara-Hernández R, Casas-González P, García-Sáinz JA. Roles of c-Src in alpha1B-adrenoceptor phosphorylation and desensitization. Auton Autacoid Pharmacol 2008;28:29-39. [PubMed]
- Harding MA, Theodorescu D. RhoGDI signaling provides targets for cancer therapy. Eur J Cancer 2010;46:1252-9. [PubMed]
- Ma L, Xu G, Sotnikova A, et al. Loss of expression of LyGDI (ARHGDIB), a rho GDP-dissociation inhibitor, in Hodgkin lymphoma. Br J Haematol 2007;139:217-23. [PubMed]
- Niu H, Li H, Xu C, et al. Expression profile of RhoGDI2 in lung cancers and role of RhoGDI2 in lung cancer metastasis. Oncol Rep 2010;24:465-71. [PubMed]
- Abiatari I, DeOliveira T, Kerkadze V, et al. Consensus transcriptome signature of perineural invasion in pancreatic carcinoma. Mol Cancer Ther 2009;8:1494-504. [PubMed]
- Cho HJ, Baek KE, Park SM, et al. RhoGDI2 expression is associated with tumor growth and malignant progression of gastric cancer. Clin Cancer Res 2009;15:2612-9. [PubMed]
- Stevens EV, Banet N, Onesto C, et al. RhoGDI2 antagonizes ovarian carcinoma growth, invasion and metastasis. Small GTPases 2011;2:202-10. [PubMed]
- Goto T, Takano M, Sakamoto M, et al. Gene expression profiles with cDNA microarray reveal RhoGDI as a predictive marker for paclitaxel resistance in ovarian cancers. Oncol Rep 2006;15:1265-71. [PubMed]
- Cho HJ, Baek KE, Park SM, et al. RhoGDI2 confers gastric cancer cells resistance against cisplatin-induced apoptosis by upregulation of Bcl-2 expression. Cancer Lett 2011;311:48-56. [PubMed]
- Zheng Z, Li J, He X, et al. Involvement of RhoGDI2 in the resistance of colon cancer cells to 5-fluorouracil. Hepatogastroenterology 2010;57:1106-12. [PubMed]
- Zheng Z, He XY, Li JF, et al. RhoGDI2 confers resistance to 5-fluorouracil in human gastric cancer cells. Oncol Lett 2013;5:255-60. [PubMed]
- Kobinger GP, Weiner DJ, Yu QC, et al. Filovirus-pseudotyped lentiviral vector can efficiently and stably transduce airway epithelia in vivo. Nat Biotechnol 2001;19:225-30. [PubMed]
- Medina MF, Kobinger GP, Rux J, et al. Lentiviral vectors pseudotyped with minimal filovirus envelopes increased gene transfer in murine lung. Mol Ther 2003;8:777-89. [PubMed]
- Watson DJ, Kobinger GP, Passini MA, et al. Targeted transduction patterns in the mouse brain by lentivirus vectors pseudotyped with VSV, Ebola, Mokola, LCMV, or MuLV envelope proteins. Mol Ther 2002;5:528-37. [PubMed]
- Limberis MP, Bell CL, Heath J, et al. Activation of transgene-specific T cells following lentivirus-mediated gene delivery to mouse lung. Mol Ther 2010;18:143-50. [PubMed]
- Hendrickson B, Senadheera D, Mishra S, et al. Development of lentiviral vectors with regulated respiratory epithelial expression in vivo. Am J Respir Cell Mol Biol 2007;37:414-23. [PubMed]
- Black JL, Oliver BG, Roth M. Molecular mechanisms of combination therapy with inhaled corticosteroids and long-acting beta-agonists. Chest 2009;136:1095-100. [PubMed]
- Soh UJ, Dores MR, Chen B, et al. Signal transduction by protease-activated receptors. Br J Pharmacol 2010;160:191-203. [PubMed]
- Violin JD, DiPilato LM, Yildirim N, et al. beta2-adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. J Biol Chem 2008;283:2949-61. [PubMed]
- Baker JG. The selectivity of beta-adrenoceptor agonists at human beta1-, beta2- and beta3-adrenoceptors. Br J Pharmacol 2010;160:1048-61. [PubMed]
- Sears MR, Lötvall J. Past, present and future--beta2-adrenoceptor agonists in asthma management. Respir Med 2005;99:152-70. [PubMed]
- Iyer V, Tran TM, Foster E, et al. Differential phosphorylation and dephosphorylation of beta2-adrenoceptor sites Ser262 and Ser355,356. Br J Pharmacol 2006;147:249-59. [PubMed]
- Giembycz MA, Newton R. Beyond the dogma: novel beta2-adrenoceptor signalling in the airways. Eur Respir J 2006;27:1286-306. [PubMed]
- Penn RB, Panettieri RA Jr, Benovic JL. Mechanisms of acute desensitization of the beta2AR-adenylyl cyclase pathway in human airway smooth muscle. Am J Respir Cell Mol Biol 1998;19:338-48. [PubMed]


