Total arch replacement and frozen elephant trunk for type A aortic dissection after Bentall procedure in Marfan syndrome
Introduction
Aortic root dilatation is the most common cardiovascular manifestation in patients with Marfan syndrome (MFS) because of their inherently weakened aortic wall (1). Aortic root aneurysm and aortic dissection followed by aortic rupture are the major causes of morbidity and mortality. Prophylactic aortic root surgery has been effective in preventing acute dissection and rupture (2-4), with low operative mortality and excellent long-term outcomes (2,3,5,6). However, many patients develop aneurysm or dissection beyond the aortic root after prophylactic root surgery (1,4,6). According to recent data from the International Registry on Acute Aortic Dissection (4), acute type A dissection (TAAD) in Marfan patients is usually managed with an emergency root and/or ascending replacement, which is associated with low operative risks but leaves the aortic arch and distal aorta untreated (1). Consequently, reoperation is required to manage the patent false lumen (FL), seen in 29–78% of patients (7-12), and the expanding aneurysm in the distal aorta (7,13,14). In addition, reoperation for arch dissection was required in 40.9–49% of patients (15,16). As a result, the more extensive total arch replacement (TAR) (15) or in combination with the frozen elephant trunk (FET) has been advocated as an approach to TAAD involving the aortic arch in patients with MFS during initial surgical treatment (17-19), despite concerns on the mortality and paraplegia risks and limited imaging follow-up (20,21). Although our previous study has proved the efficacy and durability of TAR + FET for TAAD in MFS (22), data is scarce regarding the long-term outcomes of this technique for TAAD in Marfan patients who have undergone a Bentall operation previously. The present study aims to evaluate the early and long-term outcomes of the FET + TAR techniques for such patients, including mortality and morbidity, late survival and reoperation, changes in the distal aorta, and risk factors for late adverse events.
Methods
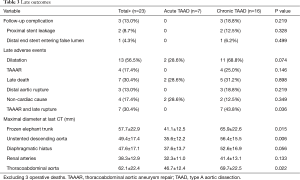
Between April 2003 and August 2015, we performed TAR and FET for 26 patients who developed TAAD involving the aortic arch after a prior Bentall procedure. Patients were divided into two groups according to acuity, acute in 8 (30.8%, all new dissections) and chronic in 18 patients (69.2%, 15 residual and 3 new dissections). Indication for prior Bentall was TAAD in 15 (57.7%) and root aneurysm in 11 (42.3%). The diagnosis of MFS was confirmed by the Ghent and/or revised Ghent criteria (23,24). Mean age at TAR + FET was 36.9±9.7 years (range, 17–50 years). Hypertension was seen in 13 (50.0%) patients. The interval between Bentall and FET averaged 6.4±5.8 years, which was significantly longer in patients with acute TAAD (10.3±6.3 vs. 4.6±4.9 years; P=0.021). The preoperative clinical profiles are listed in Table 1.
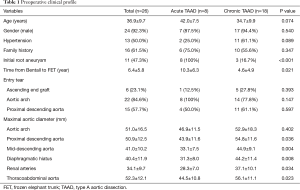
Full table
All dissections were confirmed by computed tomographic angiography (CTA). Before the FET, the mean maximal diameter (DMax) of the aortic arch was 51.0±16.5 mm, which was larger in chronic TAAD patients, but without significant difference (52.9±18.3 vs. 46.9±11.5 mm; P=0.402); the mean DMax in the chronic group were significantly larger compared to acute patients at the proximal descending aorta (DA) (54.8±11.6 vs. 43.9±11.6 mm; P=0.036), diaphragmatic hiatus (DH) (44.2±11.4 vs. 31.3±8.0 mm; P=0.008) and renal arteries (RA) (37.1±10.1 vs. 28.3±7.0 mm, P=0.034), respectively. Coronary artery endoleak was detected in 4 patients with chronic TAAD.
Surgical technique
Our selection criteria for TAR + FET for TAAD in Marfan patients are as follows: (I) dissection with an entry tear located in the transverse arch or DA; (II) aneurysm of the aortic arch or distal aorta (>40 mm in diameter); (III) dissection, aneurysm, or occlusion of the brachiocephalic arteries.
All patients underwent this reoperative procedure through a redo sternotomy. Before sternotomy, cardiopulmonary bypass (CPB) was instituted first by cannulation of the femoral artery and vein. The aortic arch, proximal DA and arch vessels were dissected on CPB before circulatory arrest. The surgical technique, known as the Sun’s procedure, has been previously described in detail (17,25-27). Briefly, right axillary artery cannulation is used for CPB and unilateral selective antegrade cerebral perfusion under moderate hypothermic circulatory arrest at 25 °C is utilized. The aortic arch is transected between the left carotid and left subclavian arteries (26). After the FET (Cronus®, MicroPort Medical, Shanghai, China) is deployed in the DA, TAR is performed with a 4-branched vascular graft (Maquet, Rastatt, Germany). To minimize the time of cerebral, myocardial and spinal cord ischemia, distal reperfusion is initiated once the distal anastomosis is completed, and the left carotid artery is reconstructed first (after which rewarming is started and the brain is perfused bilaterally), followed by the ascending aorta (to resume myocardial perfusion), then the left subclavian artery (LSA), and finally, the innominate artery. Proximal procedures (coronary artery anastomotic leak repair and mitral valve surgery) are performed during the cooling phase.
Patient follow-up
Operative survivors were recommended to take β-blockers as routine medication after discharge, even if they had normal blood pressure.
CTA of the entire aorta was performed at discharge or 1, 3 and 6 months, 1 year and annually henceforth to assess the FL, TL, maximal aortic size (DMax) and growth rate, and complications (endoleak, migration, etc.). The statuses of the FL were classified as: completely thrombosed (obliterated) if no flow was present; partially thrombosed if both flow and thrombus were present; and patent if flow was present in the absence of thrombus (28). The maximum aortic diameter (DMax) was measured from the outer contours of the aortic wall in the axial plane and analyzed at four different distal aortic levels: the stented proximal DA (FET), the unstented DA, the DH and renal arteries. The segmental aortic growth rate was assessed in patients who had ≥2 CT scans postoperatively with an interval of at least 6 months apart. The growth rate was calculated by dividing the diameter differences between the initial and last CTs by the interval in between (mm/year). Dilatation was defined as a maximum diameter ≥50 mm (45 mm in patients with a family history of aortic surgery or rupture) or an average growth rate of ≥5 mm/year.
Follow-up was complete in 100% (23/23) by June 2017 for a mean duration of 5.1±2.3 years (range, 0.9–11.2 years).
Statistical analysis
Statistical analysis was performed using SPSS for Windows 19.0 (SPSS Inc., Chicago, IL, USA), Stata 15.1 for Mac (StataCorp, College Station, TX, USA) and Prsim 7.0d for Mac (GraphPad, La Jolla, CA, USA). Data are expressed as the mean ± standard deviation or number (percentage) and were compared using the Student t-test or Pearson chi-square test for normal distributions, and the Mann-Whitney U test for abnormal distributions, as appropriate. Risk factors for distal aortic dilatation, late distal adverse events (reoperation and rupture) were identified with Cox regression model. Variables considered in multivariate analysis included age, hypertension, preoperative descending aortic diameter, FL patency, annual growth rate and maximum diameter of thoracoabdominal aorta. Survival and freedom from dilatation and reoperation were estimated using the Kaplan-Meier method, and intergroup comparisons made with the log-rank test. Competing risks of death and reoperation were analyzed with the Fine and Gray proportional hazards model. All statistical tests were 2-sided and a P value of <0.05 was considered statistically significant.
Results
Operative data
The entry tears were located in the aortic arch in 22 (84.6%), proximal DA in 15 (57.7%), and the ascending aorta (distal to the Bentall graft) in 6 (23.1%) patients. Multiple entry tears were seen in 13 (50%) patients. The extent of dissection was to the iliac artery in 24 (92.3%) patients and to the distal descending thoracic aorta in 2 (7.7%) patients.
An entry tear in the arch was found in 100% (8/8) patients of the acute group (all with prior root aneurysm) and in 77.8% (14/18) patients of the chronic group (P=0.147). In the chronic group, there were 15 residual dissections from previous TAAD; among these, 1 (6.7%) patient underwent FET for an entry tear in the arch 9 years after Bentall. In his prior Bentall procedure, no intimal tear was found in the arch and DA. The other 14 (14/15, 93.3%) patients had intimal tears located in the aortic arch or proximal DA that were not resected during initial surgery and underwent FET for dilation at mean 2.3±1.3 years, with an annual growth rate of 8.6 mm in the DA and 13 mm in the DH.
The times of CPB, cross-clamp and cerebral perfusion were 184±42, 88±32, 23±6 minutes, respectively. CPB time was longer in chronic patients (191.3±37.2 vs. 168.8±49.4 minutes, P=0.208), but without significant difference between two groups. Concomitant procedures included mitral valve surgery in 2 (7.7%), ascending aortic to femoral bypass in 1 (3.8%), and coronary leakage repair in 4 (15.4%) patients, respectively.
Operative mortality and morbidity
The operative mortality rate was 11.5% (3 of 26), occurring in 1 patient with acute TAAD and 2 with chronic TAAD (12.5% vs. 11.1%, P=0.919). All 3 expired patients were with a root aneurysm; 2 had a markedly dilated arch (65 and 150 mm in size) with a CPB time of 246 and 153 minutes, respectively. The cause of death was multiorgan failure, hemorrhagic stroke and distal aortic rupture, in 1 each (3.8%). No spinal cord injury occurred. Lower limb ischemia and reexploration for bleeding occurred in 1 patient each (3.8%) (Table 2).
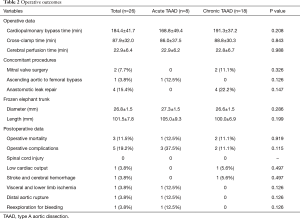
Full table
Distal aortic dilation and reoperation
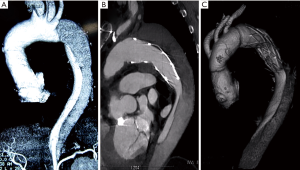
Obliteration of the false lumen was observed in 95.7%, 56.5% and 26.1% at the levels of FET, DA and DH, respectively. Patients with acute TAAD had higher rates of FL thrombosis (Figure 1) at the levels of FET (100% vs. 93.8%, P=0.499) and DA (71.4% vs. 50.0%, P=0.340); this difference was significant at the level of DH (57.1% vs. 12.5%, P=0.025).
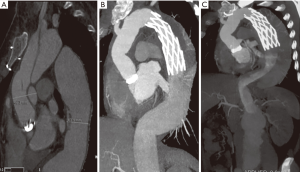
On the latest follow-up CT, the DMax of distal aorta in the chronic group were greater compared to patients with acute TAAD (Figure 2), which differed significantly at the levels of FET (65.9±22.6 vs. 41.1±12.5 mm, P=0.015) and DA (56.4±15.5 vs. 35.6±12.2 mm, P=0.006). However, the DMaxs did not differ significantly at the levels of DH (52.6±16.9 vs. 37.6±13.7 mm, P=0.056) and RA (41.4±13.1 vs. 32.3±11.0 mm, P=0.133).
Distal aortic dilatation occurred in 13 patients during follow-up, 2 in the acute and 11 in the chronic groups (28.6% vs. 68.8%, P=0.074). In the chronic group, 3 patients died of distal aortic rupture and 4 underwent an open thoracoabdominal aortic repair (TAAAR). The median interval from FET to TAAAR was 0.5 years. The average DMax at TAAAR was 83.2±21.2 mm (range, 70–115 mm).
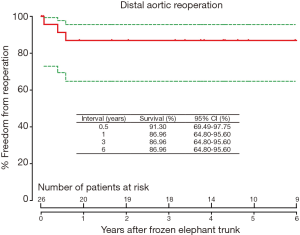
The freedom from reoperation on the distal aorta was 86.96% [95% confidence interval (CI), 64.8–95.6%] at 1, 3 and 6 years, respectively (Figure 3); and 72.5% (95% CI, 33.8–90.9%) at 7 years.
Late survival
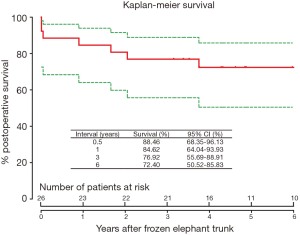
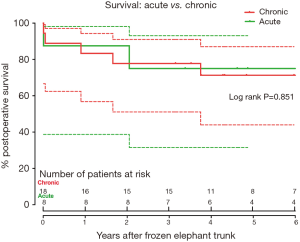
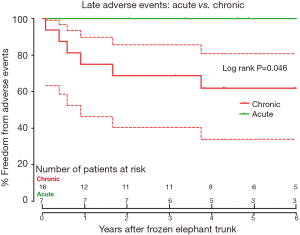
Late death occurred in 7 patients. The cause of death was distal aortic rupture from residual dissection in 3 patients, all in the chronic TAAD group, and non-cardiac reasons in 4 patients (Table 3). Survival was 84.6% (95% CI, 64.0–93.9%), 76.9% (95% CI, 55.7–88.9%) and 72.4% (95% CI, 50.5–85.8%) at 1, 3, and 6 years, respectively (Figure 4). At 6 years after FET, survival did not differ between two groups (75.0% vs. 71.3%, P=0.851; Figure 5), while freedom from distal aortic reoperation and rupture were significantly higher in the acute group at 6 years (100% vs. 61.9%, P=0.046; Figure 6).
Full table
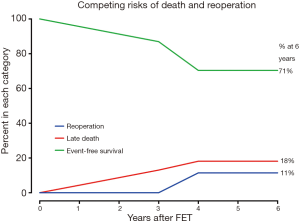
In competing risks analysis, the incidence of was 18% for late death, 11% for distal aortic reoperation, and 71% for event-free survival at 6 years after FET (Figure 7).
Risk factors for late adverse events
Hypertension was the sole risk factor for distal aortic dilatation [hazard ratio (HR) =7.271; 95% CI, 1.814–29.143; P=0.005]. Risk factors for late adverse events (aortic rupture and reoperation) were hypertension (HR =6.712; 95% CI, 1.201–37.503; P=0.030) and age <35 years (HR =6.760; 95% CI, 1.154–39.587; P=0.034) (Table 4).

Full table
Discussion
In surgical management of type A dissection for patients with MFS, the extent of surgery (limited proximal versus extensive total arch repair) has to be balanced between the operative mortality risk and the unique challenges in those patients, such as residual and new dissections, and expanding aneurysms in downstream aorta (1,14,16). This is particularly important in the surgical management of Marfan patients sustaining TAAD after a Bentall procedure. Besides the resternotomy and adhesions, technical complexities in this reoperative setting include the multiple residual intimal tears in the arch or proximal DA, usually with a huge arch aneurysm, and significantly dilated proximal distal aorta. Dissecting the brachiocephalic arteries, especially the left subclavian is much more technically demanding. A recent study has found that at the time of initial repair for TAAD in MFS, a more aggressive surgical approach seems to be superior to limited repair (29). However, there is scarce data on the long-term outcomes of such extensive repair for TAAD in such patients (19,22,30).
The current cohort expanded the special subset of MFS patients in our experience (17,22,31), who, following a prior Bentall procedure, developed TAAD involving the arch, which was repaired in a reoperative setting with the extensive FET technique. Compared to other MFS patients in our experience (22), the proximal procedure (Bentall) had already been performed in this cohort, which avoids longer CPB time, a risk factor for operative mortality (32,33). In this series, operative mortality was 11.5%; at 6 years late death occurred in 18%, 11% underwent a distal aortic reoperation, and 71% of patients were alive without reoperation. These early and late outcomes are comparable or superior to most series of arch reoperations (1,15,34). In the report of Bachet and associates (15), 35 patients underwent 45 reoperations and 7 died before discharge (13%). Girdauskas and colleagues (1) reported a cohort of 15 Marfan patients undergoing reintervention after root surgery and the mortality rate was 6.7%. However, long-term data is lacking in both reports. These results of the current study show that the FET technique is a feasible and efficacious approach to TAAD after a prior Bentall procedure in Marfan patients in a reoperative setting.
The incidence of reoperations is significantly higher in patients who presented initially with acute TAAD than in those with dilatation only (1,34,35). In the present study, the entry tear was not resected during previous surgery in 15 of 26 patients, which exposed them to the risk of chronic dissection with residual tears, aggravating dilation of distal aortic segments (36). MFS patients with chronic dissection tend to have a very narrowed true lumen, which is compressed by an enlarged false lumen as a result of residual dissections untreated in the prior Bentall operation. And many of them have a thoracoabdominal aortic aneurysm, as evidenced by the significantly larger maximal distal aortic sizes before surgery. These chronic dissection patients may be at the more severe end of the disease spectrum (22). Following the FET, the rates of false lumen obliteration were significantly lower across the FET, quick dilation of downstream aorta, re-dissection and rupture and the need for distal reoperation are not unexpected in these patients. This largely accounts for the lower freedom from late rupture and distal reoperation in the chronic group as compared to the patients with acute TAAD.
When should the aortic arch be replaced in Marfan patients and whether TAR should be performed during initial surgery are controversial (1,7,8,11,15). Few reports have addressed the issue of the extent of the aortic replacement (15,16,35). In the present study, the significantly longer duration between the two procedures in acute TAAD group proved the durability of the Bentall operation for patients with root aneurysm. Although it is not clear how and when a new dissection would happen to effect on primarily nontreated aortic segments, prophylactic arch replacement is not necessary during initial root replacement (16). In this study, favorable long-term outcomes have been achieved using TAR + FET for the root aneurysm patients, with no late rupture and reoperation. To decrease the mortality risk of reoperation, we recommend that secondary TAR + FET be performed for root aneurysm patients when the maximal size of the aortic arch and/or proximal DA reached 4.0 cm or when the growth rate exceeds 5 mm/year.
Aortic dissection during the initial root procedure has been identified as a significant risk factor for distal aortic reoperations (1,12). Bachet et al. (15) reported that 73% of secondary TAR and 53% of TAAAR were required after Bentall procedure in patients with initial TAAD, and therefore proposed more aggressive approach toward the aortic arch during initial surgery. However, it remains unclear how long the interval is safe enough between the root and arch surgery to prevent rupture of the distal aneurysm. In some patients with residual dissection and significantly dilated arch and thoracoabdominal aorta, the choice of surgical access would be another dilemma. Based on our experience (17,22,31) and that of others (1,16,34,35), we recommended TAR + FET be performed during initial surgery if dissection involves the aortic arch in MFS patients. This approach may at least eliminate the need for an additional operation on the arch and proximal DA via a repeat median sternotomy in some patients. In addition, the Cronus FET has an extra centimeter of Dacron sewing cuff at the distal end, which facilitates manipulations during second-stage repair or reoperations should this be required (37).
The present study identified hypertension as the sole risk factor for distal aortic dilatation and one of two predictors of late adverse events. Therefore, antihypertensive therapy and close monitoring of the entire aorta with CT are essential for the optimal management of all patients with MFS (38). This is of particular importance to those who underwent a Bentall procedure for initial TAAD with unresected intimal tears in the arch and DA.
Study limitations
The limitations of this study include a small sample size and the lack of a control group. Additionally, this is a retrospective analysis of a single-center experience with a mixture of different acuity and etiology conditions. The patients are extremely young even in view of the fact that they are Marfan, and therefore, an average follow-up period of 5.1 years is not long enough. Due to the large case volume at our center, the recommendations we made may not be directly transferable to other centers.
Conclusions
The TAR and FET technique was feasible and efficacious for TAAD following previous Bentall procedure in patients with MFS. Early and late survival did not differ with acute and chronic dissections, while freedom from late rupture and reoperation is significantly higher in patients with acute TAAD. Patients with hypertension and aged <35 years are at higher risk for late distal aortic dilation, reoperation and death.
Acknowledgements
Funding: This study was supported by the Special Research Fund for Public Health and Welfare (No. 201402009).
Footnote
Conflicts of Interest: Abstract of this work was presented at the American Association for Thoracic Surgery Aortic Symposium 2018, New York, NY, USA, April 26–27, 2018.
Ethical Statement: This study was approved by the Ethic Committees of Beijing Anzhen Hospital of Capital Medical University and Fu Wai Hospital Chinese Academy of Medical Sciences (No. 2013013X).
References
- Girdauskas E, Kuntze T, Borger MA, et al. Distal aortic reinterventions after root surgery in Marfan patients. Ann Thorac Surg 2008;86:1815-9. [Crossref] [PubMed]
- Finkbohner R, Johnston D, Crawford ES, et al. Marfan syndrome. Long-term survival and complications after aortic aneurysm repair. Circulation 1995;91:728-33. [Crossref] [PubMed]
- Ades L. CSANZ Cardiovascular Genetics Working Group. Guidelines for the diagnosis and management of Marfan syndrome. Heart Lung Circ 2007;16:28-30. [Crossref] [PubMed]
- de Beaufort HW, Trimarchi S, Korach A, et al. Aortic dissection in patients with Marfan syndrome based on the IRAD data. Ann Cardiothorac Surg 2017;6:633-41. [Crossref] [PubMed]
- Gott VL, Greene PS, Alejo DE, et al. Replacement of the aortic root in patients with Marfan's syndrome. N Engl J Med 1999;340:1307-13. [Crossref] [PubMed]
- Cameron DE, Alejo DE, Patel ND, et al. Aortic root replacement in 372 Marfan patients: Evolution of operative repair over 30 years. Ann Thorac Surg 2009;87:1344-9; discussion 9-50. [Crossref] [PubMed]
- Fattouch K, Sampognaro R, Navarra E, et al. Long-term results after repair of type A acute aortic dissection according to false lumen patency. Ann Thorac Surg 2009;88:1244-50. [Crossref] [PubMed]
- Fattori R, Bacchi-Reggiani L, Bertaccini P, et al. Evolution of aortic dissection after surgical repair. Am J Cardiol 2000;86:868-72. [Crossref] [PubMed]
- Halstead JC, Meier M, Etz C, et al. The fate of the distal aorta after repair of acute type A aortic dissection. J Thorac Cardiovasc Surg 2007;133:127-35. [Crossref] [PubMed]
- Park KH, Lim C, Choi JH, et al. Midterm change of descending aortic false lumen after repair of acute type I dissection. Ann Thorac Surg 2009;87:103-8. [Crossref] [PubMed]
- Uchida N, Shibamura H, Katayama A, et al. Operative strategy for acute type A aortic dissection: ascending aortic or hemiarch versus total arch replacement with frozen elephant trunk. Ann Thorac Surg 2009;87:773-7. [Crossref] [PubMed]
- Schoenhoff FS, Carrel TP. Re-interventions on the thoracic and thoracoabdominal aorta in patients with Marfan syndrome. Ann Cardiothorac Surg 2017;6:662-71. [Crossref] [PubMed]
- Sakaguchi G, Komiya T, Tamura N, et al. Patency of distal false lumen in acute dissection: Extent of resection and prognosis. Interact Cardiovasc Thorac Surg 2007;6:204-7. [Crossref] [PubMed]
- Schoenhoff FS, Jungi S, Czerny M, et al. Acute aortic dissection determines the fate of initially untreated aortic segments in Marfan syndrome. Circulation 2013;127:1569-75. [Crossref] [PubMed]
- Bachet J, Larrazet F, Goudot B, et al. When should the aortic arch be replaced in Marfan patients? Ann Thorac Surg 2007;83:S774-9; discussion S85-90.
- Tagusari O, Ogino H, Kobayashi J, et al. Should the transverse aortic arch be replaced simultaneously with aortic root replacement for annuloaortic ectasia in Marfan syndrome? J Thorac Cardiovasc Surg 2004;127:1373-80. [Crossref] [PubMed]
- Sun L, Qi R, Chang Q, et al. Surgery for Marfan patients with acute type A dissection using a stented elephant trunk procedure. Ann Thorac Surg 2008;86:1821-5. [Crossref] [PubMed]
- Uchida N, Katayama A, Kuraoka M, et al. Extended aortic repair using frozen elephant trunk technique for Marfan syndrome with acute aortic dissection. Ann Thorac Cardiovasc Surg 2013;19:279-82. [Crossref] [PubMed]
- Shrestha M, Martens A, Kaufeld T, et al. Single-centre experience with the frozen elephant trunk technique in 251 patients over 15 years. Eur J Cardiothorac Surg 2017;52:858-66. [Crossref] [PubMed]
- Weiss G, Santer D, Dumfarth J, et al. Evaluation of the downstream aorta after frozen elephant trunk repair for aortic dissections in terms of diameter and false lumen status. Eur J Cardiothorac Surg 2016;49:118-24. [Crossref] [PubMed]
- Dohle DS, Tsagakis K, Janosi RA, et al. Aortic remodelling in aortic dissection after frozen elephant trunk. Eur J Cardiothorac Surg 2016;49:111-7. [Crossref] [PubMed]
- Ma WG, Zhang W, Zhu JM, et al. Long-term outcomes of frozen elephant trunk for type A aortic dissection in patients with Marfan syndrome. J Thorac Cardiovasc Surg 2017;154:1175-89.e2. [Crossref] [PubMed]
- De Paepe A, Devereux RB, Dietz HC, et al. Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet 1996;62:417-26. [Crossref] [PubMed]
- Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet 2010;47:476-85. [Crossref] [PubMed]
- Ma WG, Zheng J, Liu YM, et al. Dr. Sun's procedure for type A aortic dissection: Total arch replacement using tetrafurcate graft with stented elephant trunk implantation. Aorta (Stamford) 2013;1:59-64. [Crossref] [PubMed]
- Ma WG, Zhu JM, Zheng J, et al. Sun's procedure for complex aortic arch repair: total arch replacement using a tetrafurcate graft with stented elephant trunk implantation. Ann Cardiothorac Surg 2013;2:642-8. [PubMed]
- Sun LZ, Ma WG, Zhu JM, et al. Sun's procedure for chronic type A aortic dissection: total arch replacement using a tetrafurcate graft with stented elephant trunk implantation. Ann Cardiothorac Surg 2013;2:665-6. [PubMed]
- Peterss S, Mansour AM, Ross JA, et al. Changing pathology of the thoracic aorta from acute to chronic dissection: Literature review and insights. J Am Coll Cardiol 2016;68:1054-65. [Crossref] [PubMed]
- Rylski B, Bavaria JE, Beyersdorf F, et al. Type A aortic dissection in Marfan syndrome: Extent of initial surgery determines long-term outcome. Circulation 2014;129:1381-6. [Crossref] [PubMed]
- Iafrancesco M, Goebel N, Mascaro J, et al. Aortic diameter remodelling after the frozen elephant trunk technique in aortic dissection: results from an international multicentre registry. Eur J Cardiothorac Surg 2017;52:310-8. [Crossref] [PubMed]
- Sun L, Li M, Zhu J, et al. Surgery for patients with Marfan syndrome with type A dissection involving the aortic arch using total arch replacement combined with stented elephant trunk implantation: The acute versus the chronic. J Thorac Cardiovasc Surg 2011;142:e85-91. [Crossref] [PubMed]
- Ma WG, Zheng J, Zhang W, et al. Frozen elephant trunk with total arch replacement for type A aortic dissections: Does acuity affect operative mortality? J Thorac Cardiovasc Surg 2014;148:963-70; discussion 970-2. [Crossref] [PubMed]
- Ma WG, Zhang W, Wang LF, et al. Type A aortic dissection with arch entry tear: Surgical experience in 104 patients over a 12-year period. J Thorac Cardiovasc Surg 2016;151:1581-92. [Crossref] [PubMed]
- Malvindi PG, van Putte BP, Sonker U, et al. Reoperation after acute type A aortic dissection repair: a series of 104 patients. Ann Thorac Surg 2013;95:922-7. [Crossref] [PubMed]
- Carrel T, Beyeler L, Schnyder A, et al. Reoperations and late adverse outcome in Marfan patients following cardiovascular surgery. Eur J Cardiothorac Surg 2004;25:671-5. [Crossref] [PubMed]
- Engelfriet PM, Boersma E, Tijssen JG, et al. Beyond the root: dilatation of the distal aorta in Marfan's syndrome. Heart 2006;92:1238-43. [Crossref] [PubMed]
- Ma WG, Zheng J, Sun LZ, et al. Open stented grafts for frozen elephant trunk technique: technical aspects and current outcomes. Aorta (Stamford) 2015;3:122-35. [Crossref] [PubMed]
- Bin Mahmood SU, Velasquez CA, Zafar MA, et al. Medical management of aortic disease in Marfan syndrome. Ann Cardiothorac Surg 2017;6:654-61. [Crossref] [PubMed]