Thoracoscopic segmentectomy: hybrid approach for clinical stage I non-small cell lung cancer
Introduction
Recently, sublobar resection including radical segmentectomy (RS) for clinical stage I small-sized non-small-cell primary lung cancer has become an important issue, and use of this procedure has been increasing (1-3). Video-assisted thoracic surgery (VATS) segmentectomy is an ideal surgical procedure (4). Cao et al. (5) reported a systematic meta-analytical review comparing sublobar resection with lobectomy in non-small cell lung cancer. They found no significant difference in overall survival (OS) or disease-free survival (DFS) in the intentional segmentectomy group compared with lobectomy, but significantly worse outcomes for segmentectomy in the compromised group. We therefore consider meticulous anatomical segmentectomy for maintaining tumor surgical margins as the most important issue for obtaining desirable outcomes in lung cancer.
Recently, minimally invasive surgical approaches, typified by VATS, have been developed. Swanson et al. (6) defined VATS lobectomy: “to encompass a true anatomic lobectomy with individual ligation of lobar vessels and bronchus as well as hilar lymph node dissection or sampling using the video screen for guidance, two or three ports, and no retractor use or rib spreading.” We call this procedure “thoracoscopic VATS”. Conversely, Okada et al. (7) described a method for a mini-thoracotomy that spares muscles, uses a 4- to 10-cm incision, and includes video assistance for direct visualization during lung surgery. They have named this method “hybrid VATS”. In this article, we demonstrate the validity of hybrid VATS segmentectomy.
Methods
Patients
The Institutional Review Board at the Graduate School of Medicine, Gifu University approved this study (approval no. 29-100). Of 686 patients who underwent lobectomy or segmentectomy from 2004 to 2016 (434 men, 252 women; mean age, 69.1±9.6 years) with clinical stage I primary lung cancer, lobectomy was performed in 561 patients, and segmentectomy was performed in 125 patients. Of these 125 patients, 62 (49.6%) underwent intensively RS for pure grand glass nodule (GGN), more than 50% GGN and <2 cm in diameter, solid tumors <10 mm in diameter, or as part of a Japanese clinical study [the Japan Clinical Oncology Group/West Japan Oncology Group (JCOG0802/WJOG4607L)] (8). The remaining 63 (50.4%) patients underwent palliative segmentectomy (PS).
Hybrid VATS procedure
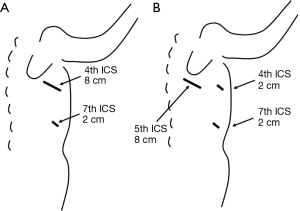
For our hybrid VATS procedure, we used 2-cm port is placed in the 7th intercostal spaces (ICS) along the anterior axillary line, plus a mini-thoracotomy to spare muscles with a metal retractor that was minimally opened. When resecting the middle or upper lobe, we made an incision that was 4–8 cm in length in the 4th intercostal space along the anterior axillary line (Figure 1A). When removing the lower lobe, the incision was made in the 5th intercostal space along the posterior axillary line with two 2-cm ports, which were placed in the 4th and 7th ICS along the anterior axillary line (Figure 1B). In all cases of hybrid VATS, a direct, thoracoscopic view was established (9). To approach the upper or middle lobe, the surgeon typically stands on the ventral side of the patient. A dorsal approach is needed when resecting the lower lobe. In addition, we used forceps (GEISTER Medizintechnik GmbH, Tuttlingen, Germany), electrocautery, and ultrasonic scissors (HARMONIC ACE; Ethicon Endo-Surgery, Cincinnati, OH, USA).
For detection of the intersegmental plane, we performed an original method (10). Briefly, we separated the segmental pulmonary artery and vein. We then filled the segment with pure oxygen for 5 min and immediately closed the segmental bronchus with surgical staples. After a couple of minutes, the intersegmental plane was easily detected. A stapler or electrocautery was then used along this plane for the segmentectomy.
Statistical analysis
We analyzed our data using SPSS software (version 24; SPSS, Chicago, IL, USA). The frequencies of categorical variables were assessed with the χ2 test. When the sample size was small, Fisher’s exact test was performed. OS was defined as the time from the operation until death from any cause or the last follow-up. DFS was defined as the time from the operation to the first recurrence, death from any cause, or the last follow-up. OS and DFS were examined with Kaplan-Meier curves, which were statistically analyzed using log-rank tests. P<0.05 was considered to indicate a significant difference.
Results
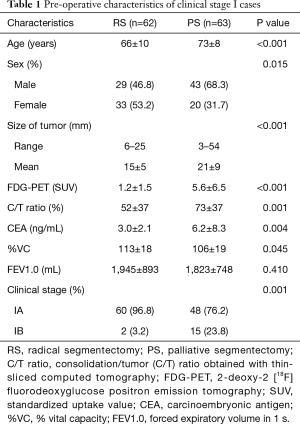
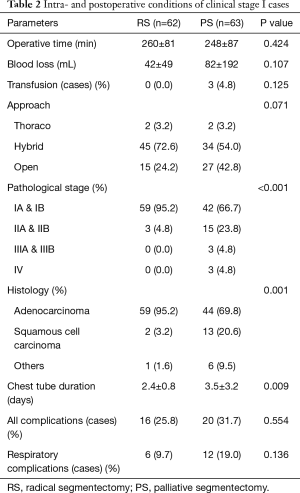
Age was significantly lower in RS cases than in PS cases (P<0.001). The proportion of female patients was significantly higher in RS cases than in PS cases (P=0.015). Tumor size was significantly smaller in PS cases (15±5 mm) than in RS cases (21±9 mm; P<0.001). Tumor standardized uptake value from 2-deoxy-2 [18F] fluorodeoxyglucose positron emission tomography was significantly lower in RS cases (1.2±1.5) than in PS cases (5.6±6.5; P<0.001). Carcinoembryonic antigen was significantly lower in RS cases (3.0±2.1 ng/mL) than in PS cases (6.2±8.3 ng/mL; P=0.004). The consolidation/tumor ratio obtained with thin-sliced computed tomography was significantly lower in RS cases (52%±37%) than in PS cases (73%±37%; P=0.001). The proportion of clinical stage IA cases was significantly higher in RS cases (96.8%) than in PS cases (76.2%; P=0.001) (Table 1). Similarly, pathological stage IA and IB cases were significantly more frequent among RS cases (95.2%) than among PS cases (66.7%; P<0.001). Adenocarcinoma was significantly more common among RS cases (95.2%) than among PS cases (69.8%; P=0.001). Duration of chest tube placement was significantly shorter in RS cases (2.4±0.8 days) than in PS cases (3.5±3.2 days; P=0.009) (Table 2).
Full table
Full table
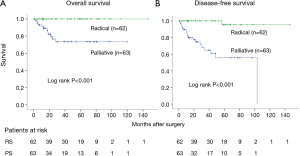
Five-year OS for clinical stage I was 100.0% in RS cases and 73.5% in PS cases (log-rank P<0.001). Five-year DFS was 95.5% and 55.7%, respectively (log-rank P<0.001) (Figure 2).
Discussion
The hybrid VATS lobectomy uses both direct and video visualization, whereas thoracoscopic VATS lobectomy uses only video visualization. A propensity-matched analysis (11) of these methods showed that the former involves shorter operation times and no differences in bleeding, chest tube duration, or complications, as well as no significant differences in OS or DFS. In another study (12), only surgical time (better with muscle-sparing thoracotomy) and hospitalization time (better with VATS) were different between groups. These researchers found no differences in the most important results, including complications after surgery, DFS, and OS. We believe that this muscle-sparing thoracotomy is similar to the hybrid VATS procedure, and that hybrid VATS is broadly equivalent to thoracoscopic VATS in terms of the reduced invasiveness.
The most critical consideration with segmentectomy is establishment of an adequate tumor margin. Khullar et al. (13) showed that lobectomy is superior to sublobar resection because insufficient lymphadenectomy and positive sublobar tumor margins often result from the latter procedure. To acquire adequate margins, Tsubota et al. (14) proposed an “extended segmentectomy” in which the line of resection is placed on the segment adjacent to the affected segment. The absence of positive margins was seen when the margin distance exceeded the maximum tumor diameter, as reported in a multicenter, prospective study (15).
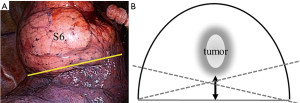
Two types of segmentectomy have been described (10): simple, in which only one intersegmental plane is resected (e.g., superior or lingual segmentectomy); and complicated, in which two or more intersegmental planes are resected (e.g., anterior segmentectomy). In the simple segmentectomy, such as a right superior (S6) segmentectomy (Figure 3A), the solid line shown in Figure 3B represents an ideal dividing intersegmental plane. However, the dashed lines should be avoided to maintain surgical margins. We suggest that this procedure can be accomplished with both thoracoscopic VATS and hybrid VATS.
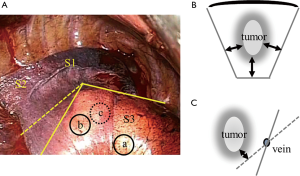
Conversely, in the complicated segmentectomy, such as a right anterior (S3) segmentectomy, when the tumor is located at “a” in Figure 4A, we can divide along the intersegmental plane represented by the solid line. However, when the tumor is located at “b” in Figure 4A, division must be made along the dashed line, extending into the adjacent segment according to the “extended segmentectomy” proposed by Tsubota et al. (14). When the tumor is located in a deep position, such as “c” in Figure 4A, more careful division to maintain the surgical margins is required. More than two intersegmental planes are present in the complicated type shown in Figure 4B. When division along with an intersegmental vein is performed (Figure 4C), the solid line represents an ideal dividing line for maintaining surgical margins. The dashed lines are insufficient to maintain adequate surgical margins. We believe that a three-dimensional, steric understanding of the intersegmental planes is required, and that hybrid VATS with direct visualization is suitable for achieving this understanding.
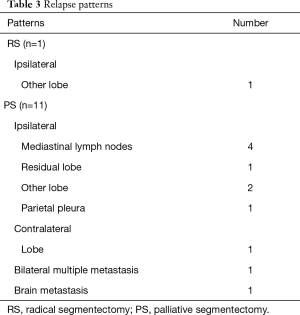
We have previously reported the feasibility of segmentectomy in non-small-cell lung cancer with GGN (16). This study is a follow-up to the previous study (16) with additional cases. In this study, we performed hybrid VATS segmentectomy in 63.2% of our cases. Only one ipsilateral other-lobe relapse was seen in RS (Table 3). Conversely, PS showed several relapse patterns. We encountered one PS case of left lower superior segmentectomy after a previous left upper lobectomy. This patient underwent local radiation to prevent a complete pneumonectomy (Table 3). Surgery in this case was performed using an open approach.
Full table
Thoracoscopic VATS segmentectomy has been reported by D’Amico (17), mainly for the simple type. Recently, Ghaly et al. (18) showed that thoracoscopic VATS segmentectomy for clinical stage I lung cancer decreases the duration of hospitalization and pulmonary complications. They demonstrated that both 5-year DFS and OS were longer with VATS than with open thoracotomy. Median surgical margins with VATS were 1.4 cm (range, 0.6–2 cm) and with thoracotomy were 1.5 cm (range, 0.8–2.7 cm). Locoregional recurrence rates were 7.7% and 12.7%, respectively. In several Japanese studies, locoregional recurrence rates have been reported as 0–5% (19-21). We suggest that determination of whether thoracoscopic VATS segmentectomy without direct visualization or rib spreading can produce sufficient tumor margins is critical, especially for complicated segmentectomy (10). To achieve meticulous surgery, the hybrid VATS segmentectomy appears sufficient for obtaining adequate tumor margins.
Conclusions
During segmentectomy, the most critical consideration is establishment of sufficient surgical margins around the cancer. Our hybrid approach that includes meticulous surgical manipulations may produce sufficient surgical margins.
Acknowledgements
The authors would like to thank Ms. Yoshimi Hayashi and Ms. Tomoko Kamiya for their secretarial support. This study was supported by grants and endowments from the Department of General and Cardiothoracic Surgery, Graduate School of Medicine, Gifu University.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Institutional Review Board at the Graduate School of Medicine, Gifu University approved this study (approval no. 29-100).
References
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Okada M. Radical sublobar resection for lung cancer. Gen Thorac Cardiovasc Surg 2008;56:151-7. [Crossref] [PubMed]
- Iwata H. Therapeutic strategy for small-sized lung cancer. Gen Thorac Cardiovasc Surg 2016;64:450-6. [Crossref] [PubMed]
- Gossot D, Lutz J, Grigoroiu M, et al. Thoracoscopic anatomic segmentectomies for lung cancer: technical aspects. J Vis Surg 2016;2:171. [Crossref] [PubMed]
- Cao C, Chandrakumar D, Gupta S, et al. Could less be more?-A systematic review and meta-analysis of sublobar resections versus lobectomy for non-small cell lung cancer according to patient selection. Lung Cancer 2015;89:121-32. [Crossref] [PubMed]
- Swanson SJ, Herndon JE 2nd, D'Amico TA, et al. Video-Assisted Thoracic Surgery Lobectomy: Report of CALGB 39802—A Prospective, Multi-Institution Feasibility Study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Okada M, Sakamoto T, Yuki T, et al. Hybrid Surgical Approach of Video-Assisted Minithoracotomy for Lung Cancer Significance of Direct Visualization on Quality of Surgery. Chest 2005;128:2696-701. [Crossref] [PubMed]
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- Yamamoto H, Shirahashi K, Matsumoto M, et al. Hybrid approach for VATS pulmonary resection. Video-assist Thorac Surg 2017;2:40. [Crossref]
- Iwata H, Shirahashi K, Mizuno Y, et al. Surgical technique of lung segmental resection with two intersegmental planes. Interact Cardiovasc Thorac Surg 2013;16:423-5. [Crossref] [PubMed]
- Iwata H, Shirahashi K, Yamamoto H, et al. Propensity score-matching analysis of hybrid video-assisted thoracoscopic surgery and thoracoscopic lobectomy for clinical stage I lung cancer. Eur J Cardiothorac Surg 2016;49:1063-7. [Crossref] [PubMed]
- Kuritzky AM, Aswad BI, Jones RN, et al. Lobectomy by Video-Assisted Thoracic Surgery vs Muscle-Sparing Thoracotomy for Stage I Lung Cancer: A Critical Evaluation of Short- and Long-Term Outcomes. J Am Coll Surg 2015;220:1044-53. [Crossref] [PubMed]
- Khullar OV, Liu Y, Gillespie T, et al. Survival After Sublobar Resection vs. Lobectomy for Clinical Stage IA Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol 2015;10:1625-33. [Crossref] [PubMed]
- Tsubota N, Ayabe K, Doi O, et al. Ongoing prospective study of segmentectomy for small lung tumors. Study Group of Extended Segmentectomy for Small Lung Tumor. Ann Thorac Surg 1998;66:1787-90. [Crossref] [PubMed]
- Sawabata N, Ohta M, Matsumura A, et al. Optimal distance of malignant negative margin in excision of nonsmall cell lung cancer: a multicenter prospective study. Ann Thorac Surg 2004;77:415-20. [Crossref] [PubMed]
- Iwata H, Shirahashi K, Mizuno Y, et al. Feasibility of segmental resection in non-small-cell lung cancer with ground-glass opacity. Eur J Cardiothorac Surg 2014;46:375-9. [Crossref] [PubMed]
- D’Amico TA. Thoracoscopic segmentectomy: technical considerations and outcomes. Ann Thorac Surg 2008;85:S716-8. [Crossref] [PubMed]
- Ghaly G, Kamel M, Nasar A, et al. Video-Assisted Thoracoscopic Surgery Is a Safe and Effective Alternative to Thoracotomy for Anatomical Segmentectomy in Patients With Clinical Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:465-72. [Crossref] [PubMed]
- Okada M, Yoshikawa K, Hatta T, et al. Is segmentectomy with lymph node assessment an alternative to lobectomy for non-small cell lung cancer of 2 cm or smaller? Ann Thorac Surg 2001;71:956-60. [Crossref] [PubMed]
- Yamashita S, Tokuishi K, Anami K, et al. Thoracoscopic segmentectomy for T1 classification of non-small cell lung cancer: a single center experience. Eur J Cardiothorac Surg 2012;42:83-8. [Crossref] [PubMed]
- Koike T, Koike T, Yamato Y, et al. Prognostic predictors in non-small cell lung cancer patients undergoing intentional segmentectomy. Ann Thorac Surg 2012;93:1788-94. [Crossref] [PubMed]


