Different microbiological and clinical aspects of lower respiratory tract infections between China and European/American countries
Introduction
Lower respiratory tract infection (LRTI) is one of the most common diseases in humans and a long-term global public health concern. Worldwide, it places considerably more strain on health budgets than do cancer, cardiovascular diseases, and malaria (1). In the United States, the incidence of LRTI and its mortality rates are higher than for any other infectious diseases. According to a 2002 WHO report, LRTIs accounted for 6.9% of all deaths in that year (2). High rates of LRTI incidence and the high medical cost involved are found worldwide, and the importance of their diagnosis and treatment is accordingly emphasized. Since the 1990s, European/American countries have developed guidelines for diagnosis and treatment of LRTI. These guidelines have been subject to regular evaluations and revisions on the basis of repeated evidence-based medical research and epidemiological investigations. The most comprehensive and influential guidelines are currently presented by the American Thoracic Society (ATS), the Infectious Diseases Society of America (IDSA), and the British Thoracic Society (BTS). Compared with European/American countries, China has diverging socioeconomic models, different medicare systems, and varying LRTI etiology and drug-resistance patterns. Therefore, simply copying the existing European/American guidelines is inappropriate and might cause serious problems in clinical practice. In recent years, large epidemiological investigations of community-acquired pneumonia (CAP), hospital-acquired pneumonia (HAP), and pulmonary mycosis were carried out in several large/middle-sized cities in China, and preliminary data on LRTI were obtained.
This paper summarized these results and discussed the differences in the microbiological and clinical features of LRTIs between China and European/American countries.
CAP
CAP is one of the most common LRTIs. The overall incidence rate is 5-11 per 1,000 people per year, accounting for 5-12% of all LRTIs (3). The pathogens causing CAP include viruses, bacteria, and other atypical pathogens. The pathogen composition is complicated and varies according to geographic area, population, and seasonal changes. The national pathogenic epidemiological investigation of CAP in 2006 indicated the following major characteristics in China.
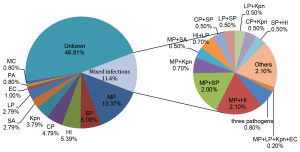
(I) The infection rate of Mycoplasma pneumoniae surpassed that of Streptococcus pneumoniae and became the leading cause of adult CAP in our country. Whereas S. pneumonia and Haemophilus influenzae are still two kinds of the most common CAP pathogens (Figure 1). A high proportion of adult cases of CAP are derived from mixed infection with bacteria and atypical pathogens (4).
Regular sputum sampling was done with most (590/610) inpatients enrolled in the study, and blood samples were taken if patients had a fever of >38.5 °C. Sputum was Gram-stained. Representative sputum originated from the lower respiratory tract was defined as that containing >25 granulocytes and
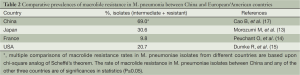
(II) High resistance to macrolides among S. pneumoniae in China is another characteristic difference compared with European/American countries. The Alexander Project Group showed that in European/American countries, rates of erythromycin resistance (resistant + intermediate resistance) in S. pneumoniae are 5). The resistance is mainly medicated by the mef(A) gene, and the common resistance phenotype is M-type (low level resistance to 14- or 15-membered macrolides and susceptibility to 16-membered macrolides and clindamycin) (6,7) (Table 1). Therefore, in those countries, macrolide antibiotics are recommended as the first-line empirical therapy in the clinic for CAP patients without risk factors (9). In contrast, in our country, the level of macrolide resistance in S. pneumoniae is higher. For example, the rate of azithromycin resistance is as high as 79.4%; mainly as constitutive resistance mediated by the erm(B) gene (cMLSB, highly resistant to erythromycin) (8,10). Considering the high divergence in drug resistance to macrolides in different areas, the CAP guidelines from ATS/IDSA (2007 edition) still recommended macrolides as the drug of first choice for previously healthy patients. However, they also pointed out that in areas with high rates of macrolide resistance, alternative antibiotics should be selected (11).

Full table
(III) Many recent studies suggest that China has the highest rates of macrolide resistance of M. pneumoniae. Since the first macrolide-resistant M. pneumoniae strain was isolated from the lower respiratory tract of a Japanese child in 2001 (12), more macrolide-resistant strains have been obtained from other countries and the prevalence is increasing annually. In 2006, this resistance rate in Japan was 30.6% (13); from 2005 to 2007, it was 9.8% in France (14); it was 3% in Germany in 2009 (15) and from 2006 to 2007, it was 20.7% in USA (16). The surveillance data from our country indicated an even worse situation (17) (Table 2). Two clinical studies in Japan showed that the pneumonia derived from macrolide-resistant strains when treated with macrolides alone did not cause deaths from significant deterioration or treatment failure. However, the recovery from fever took longer. In such cases, alternative antibiotics had to be administered when clinical symptoms aggravated (13,18). These factors lead to new difficulties for treatment of M. pneumoniae infections. In vitro culture and susceptibility testing for M. pneumoniae take long, and alternative antibiotics should be used to treat M. pneumoniae-derived pneumonia before the laboratory results become available if macrolides are not effective. For children and young adults, there are no safe and effective antibiotics that can completely replace the macrolides. Therefore, tetracycline can be used for children aged >8 years, and respiratory quinolones or tetracyclines can be used for adults. For adult patients with severe infection, respiratory quinolones alone or combined with other drugs can be used for initial treatment.
Full table
(IV) CAP caused by methicillin-resistant Staphylococcus aureus (MRSA) is still rare in China. In 1993, the first community-acquired MRSA (19) was reported in Australia. Since then, a large number of cases have also been reported in the USA, and their severity and high mortality have attracted worldwide attention (20-22). It is generally accepted that community-acquired MRSA causes more infections of the skin and soft tissues than the respiratory tract. A study from Prof. Wang H in China suggests that MRSA-derived skin and soft tissue infections only accounted for 1% of cases (23). Therefore, except for patients with a high suspicion of MRSA infection, empiric anti-MRSA drugs are not necessary.
(V) We found that the probability of infection with Escherichia coli and Klebsiellapneumoniae were significantly higher in patients aged >50 years (10). In China, the drug-resistance rates in these two bacteria are as high as 50% (24), and the approximate rate of production of extended-spectrum β-lactamase is also high (~30%) (25). The drugs recommended by foreign guidelines, for instance, respiratory quinolones alone or third-generation cephalosporins combined with macrolides or respiratory fluoroquinolones, might result in treatment failure. For elderly patients with CAP caused by E. coli, β-lactam/β-lactamase inhibitors or carbapenems should be selected.
HAP
HAP, including ventilator-associated pneumonia, is the most common nosocomial infection worldwide, with a high incidence and mortality (26). In the USA, HAP is the second most common nosocomial infection with an incidence rate of 0.5-1%, which might lengthen hospital stay by 7-9 days and increase hospital cost by >$40,000. The HAP incidence rate in patients who receive mechanical ventilation can reach 6-20% (27,28). In 2012, we carried out a nationwide multicenter, prospective epidemiological survey of HAP.
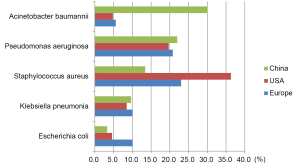
In that study, we isolated the three most common pathogenic bacteria for HAP: Acinetobacter baumannii, Pseudomonas aeruginosa, and Staph. aureus (MRSA accounted for 87.8% of Staph. aureus) (29). In European/American countries, the most common HAP bacterium is Staph. Aureus (30) (Figure 2).
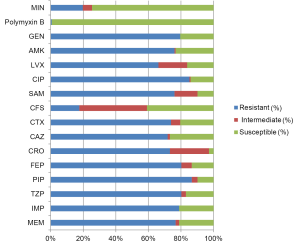
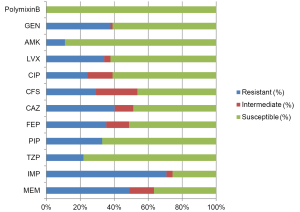
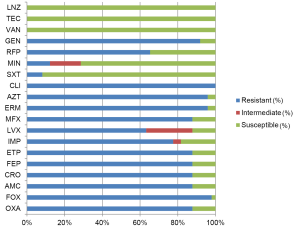
In addition to the differences in pathogen distribution, the drug resistance in non-fermentative bacteria is also more severe in China than that in European/American countries. The rates of non-susceptibility to carbapenems are nearly 80% for A. baumannii (Figure 3) and >70% for P. aeruginosa (Figure 4), indicating the reduced value of these drugs for treatment of HAP in our country. This might be associated with the uncontrolled usage of these drugs in our clinical practice. P. aeruginosa still has relatively high susceptibility to some carbapenems such as β-lactams, aminoglycosides, and quinolones. However, for A. baumannii, the drug options are relatively few and only polymyxin, sulbactam/β-lactam agents and tetracycline can be considered. The means to obtain polymyxin in China are still limited.
The most commonly isolated HAP-associated bacterium in European/American countries, Staph. aureus, is only the third most common in China, and MRSA accounts for most cases. MRSA in China still has ideal susceptibility to several anti-MRSA drugs, such as vancomycin, teicoplanin, linezolid, and tigecycline (Figure 5). The ineffectiveness of the treatments with commonly used glycopeptides in clinical practice might result from low dosage or no loading dosage. Vancomycin-resistant MRSA has not yet been found in China.
It is generally believed that the distribution of pathogenic bacteria differs significantly between early- and late-onset HAP, with the former mainly caused by susceptible bacteria and the latter by drug-resistant bacteria (31,32). In the present study, we applied a variety of tests including S. pneumoniae urinary antigen test for diagnosis. However, no significant differences were revealed between pathogens associated with early- and late-onset HAP (29). The possible explanation is that >90% of patients with either early- or late-onset HAP have been exposed to antibiotics within 90 days of the first symptom. Compared with the timing of HAP onset, the application of the antibiotics may be more relevant to the type of the pathogenic bacteria and drug resistance of pathogens. This is another difference between China and European/American countries.
Pulmonary mycosis
The number of cases of pulmonary mycosis is growing. This results from the increased number of immunocompromised hosts, and widespread application of broad-spectrum antibiotics, immunosuppressive agents, and invasive diagnostic/therapeutic technologies. Compared with bacterial LRTI, pulmonary mycosis is more difficult to treat and has poorer prognosis. Most recent foreign pulmonary mycosis guidelines are evidence-based. They are valuable for correct clinical diagnosis and proper treatment, although they might not be applicable in our country. Therefore, a national multicenter 10-year retrospective study provides more useful information for our clinicians.
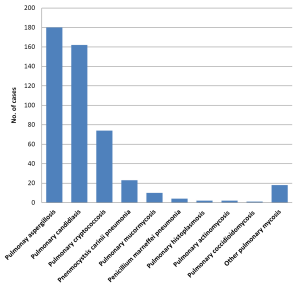
In the survey, we found in the past ten years in China that the three most common types of pulmonary mycoses were pulmonary aspergillosis, pulmonary candidiasis and pulmonary cryptococcosis (Figure 6). IDSA guidelines suggest that invasive pulmonary candidiasis is rare and lung histopathology evidence must be provided for its diagnosis. Positive sputum/bronchoalveolar lavage fluid culture cannot be used as a diagnostic criterion for pulmonary candidiasis and patients do not receive antifungal therapy in such cases (33). We included comparison of blood/pleural fluid culture with sputum culture as a criterion and found that the incidence of candidiasis was almost the same as that of aspergillosis. There were no less than 54 cases of candidiasis confirmed only by lung biopsy (34). This suggests that it is not as rare as indicated by the IDSA guidelines, which is consistent with studies in other countries. Kontoyiannis et al. performed autopsies on 676 cancer patients and found that 38% of them (254/676) had mixed pneumonia. Histopathology results showed that 14% (36/254) of cases were caused by candidiasis (35). Therefore, the description about the fungal distribution in the European/American guidelines is not applicable in China.
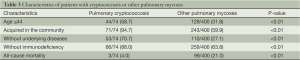
Cryptococcosis is the third leading form of pulmonary mycosis in China. Compared with other pulmonary mycoses, pulmonary cryptococcosis is characterized by high community incidence, less combined immunodeficiency or underlying diseases, and good prognosis (Table 3). Compared with foreign countries, its cure rate is higher in China (36,37), which might be related to the presence of different Cryptococcus subtypes (38).
Full table
In summary, the nationwide multicenter epidemiological studies on LRTIs revealed differences in microbiology and clinical practice between China and European/American countries. This suggests that the diagnosis/treatment guidelines should be developed based on local research results. China is a vast country and information from remote areas or medium-sized/small hospitals is still missing because of resource limitations. Further studies are needed to improve the present findings. In clinical practice, when following empirical guidelines, it is important to apply individualized treatment plans while considering the patterns of endemic pathogens, differences in hospitals.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Mizgerd JP. Lung infection-a public health priority. PLoS Med 2006;3:e76. [PubMed]
- Armstrong GL, Conn LA, Pinner RW. Trends in infectious disease mortality in the United States during the 20th century. JAMA 1999;281:61-6. [PubMed]
- Bartlett JG, Dowell SF, Mandell LA, et al. Practice guidelines for the management of community-acquired pneumonia in adults. Clin Infect Dis 2000;31:347-82. [PubMed]
- Liu Y, Chen MJ, Zhao TM, et al. Causative agent distribution and antibiotic therapy assessment among adult patients with community acquired pneumonia in Chinese urban population. BMC Infect Dis 2009;9:31. [PubMed]
- Jacobs MR, Felmingham D, Appelbaum PC, et al. The Alexander Project 1998-2000: susceptibility of pathogens isolated from community-acquired respiratory tract infection to commonly used antimicrobial agents. J Antimicrob Chemother 2003;52:229-46. [PubMed]
- Johnston NJ, De Azavedo JC, Kellner JD, et al. Prevalence and characterization of the mechanisms of macrolide, lincosamide, and streptogramin resistance in isolates of Streptococcus pneumonia. Antimicrob Agents Chemother 1998;42:2425-6. [PubMed]
- Shortridge VD, Doern GV, Brueggemann AB, et al. Prevalence of macrolide resisitance mechanisms in Streptococcus pneumoniae isolates from a multicenter antibiotic resisitance surveillance study conducted in the United States in 1994-1995. Clin Infect Dis 1999;29:1186-8. [PubMed]
- Tiemei Z, Xiangqun F, Youning L. Resistance phenotypesand genotype of erythromycin-resistant Streptococcus pneumoniae isolates in Beijing and Shenyang, China. Antimicrob Agents Chemother 2004;48:4040-1. [PubMed]
- Niederman MS, Mandell LA, Anzueto A, et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med 2001;163:1730-54. [PubMed]
- Liu YN, Chen MJ, Zhao TM, et al. A multicentre study on the pathogenic in 665 adult patients with community-acquired pneumonia in cities of China. Zhonghua Jie He He Hu Xi Za Zhi 2006;29:3-8. [PubMed]
- Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44:S27-72. [PubMed]
- Okazaki N, Narita M, Yamada S, et al. Characteristics of macrolide-resistant Mycoplasma pneumoniae strains isolated from patients and induced with erythromycin in vitro. Microbiol Immunol 2001;45:617-20. [PubMed]
- Morozumi M, Iwata S, Hasegawa K, et al. Increased macrolide resistance of Mycoplasma pneumoniae in pediatric patients with community-acquired pneumonia. Antimicrob Agents Chemother 2008;52:348-50. [PubMed]
- Peuchant O, Ménard A, Renaudin H, et al. Increased macrolide resistance of Mycoplasma pneumoniae in France directly detected in clinical specimens by real-time PCR and melting curve analysis. J Antimicrob Chemother 2009;64:52-8. [PubMed]
- Dumke R, von Baum H, Lück PC, et al. Occurrence of macrolide-resistant Mycoplasma pneumoniaestrains in Germany. Clin Microbiol Infect 2010;16:613-6. [PubMed]
- Wolff BJ, Thacker WL, Schwartz SB, et al. Detection of macrolide resistance in Mycoplasma pneumoniae by real-time PCR and high resolution melt analysis. Antimicrob Agents Chemother 2008;52:3542-9. [PubMed]
- Cao B, Zhao CJ, Yin YD, et al. High prevalence of macrolide resistance in Mycoplasma pneumoniae isolates from adult and adolescent patients with respiratory tract infection in China. Clin Infect Dis 2010;51:189-94. [PubMed]
- Suzuki S, Yamazaki T, Narita M, et al. Clinical evaluation of macrolide-resistant Mycoplasma pneumoniae. Antimicrob Agents Chemother 2006;50:709-12. [PubMed]
- Udo EE, Pearman JW, Grubb WB. Genetic analysis of community isolates of methicillin-resistant Staphylococcus aureus in Western Australia. J Hosp Infect 1993;25:97-108. [PubMed]
- Centers for Disease Control and Prevention (CDC). Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus-Minnesota and North Dakota, 1997-1999. MMWR Morb Mortal Wkly Rep 1999;48:707-10. [PubMed]
- Carrillo-Marquez MA, Hulten KG, Hammerman W, et al. USA300 is the predominant genotype causing Staphylococcus aureus septic arthritis in children. Pediatr Infect Dis J 2009;28:1076-80. [PubMed]
- Hageman JC, Uyeki TM, Francis JS, et al. Severe community-aquired pneumonia due to Staphylococcus aureus, 2003-04 influenza season. Emerg Infect Dis 2006;12:894-9. [PubMed]
- Zhao C, Liu MZ, Zhao M, et al. Characterization of community acquired staphylococcus aureus associated with skin and soft tissue infection in Beijing: high prevalence of PVL+ ST398. PLoS One 2012;7:e38577. [PubMed]
- Wang J, Li JT, Li Y. Antibacterial activities of fluoroquinolones to 2554 bacterial strains. Chin J Infect Chemother 2004;4:14-7.
- Li Y, Li JT. Comparison of detection and resisitant rates of ESBLs among Escherichia coli, Klebsiella pneumonia and Enterobacter cloacae isolates in China. Chin J Antibiot 2005;30:151-8.
- Kuti JL, Shore E, Palter M, et al. Tackling empirical antibiotic therapy for ventilator-associated pneumonia in your ICU: guidance for implementing the guidelines. Semin Respir Crit Care Med 2009;30:102-15. [PubMed]
- Guidelines for the managementofadults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388-416. [PubMed]
- American Thoracic Society, Infectious Disease Society of America. Guidelines for the managementofadults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388-416. [PubMed]
- Liu YN, Cao B, Wang H, et al. Adult hospital acquired pneumonia: a multicenter study on microbiology and clinical characteristics of patients from 9 Chinese cities. Zhonghua Jie He He Hu Xi Za Zhi 2012;35:739-46. [PubMed]
- Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis 2010;51 Suppl 1:S81-7. [PubMed]
- Hanes SD, Demirkan K, Tolley E, et al. Risk factors for late-onset nosocomial pneumonia caused by Stenotrophomonas maltophilia in critically ill trauma patients. Clin Infect Dis 2002;35:228-35. [PubMed]
- Moine P, Timsit JF, De Lassence A, et al. Mortality associated with late-onset pneumonia in the intensive care unit: results of a multi-center cohort study. Intensive Care Med 2002;28:154-63. [PubMed]
- Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009;48:503-35. [PubMed]
- Liu YN, She DY, Sun TY, et al. A multicentre retrospective study of pulmonary mycosis clinically proven from 1998 to 2007. Zhonghua Jie He He Hu Xi Za Zhi 2011;34:86-90. [PubMed]
- Kontoyiannis DP, Reddy BT, Torres HA, et al. Pulmonary candidiasis in patients with cancer: an autopsy study. Clin Infect Dis 2002;34:400-3. [PubMed]
- Aberg JA, Mundy LM, Powderly WG. Pulmonary cryptococcosis in patients without HIV infection. Chest 1999;115:734-40. [PubMed]
- Chang WC, Tzao C, Hsu HH, et al. Pulmonary cryptococcosis: comparison of clinical and radiographic characteristics in immunocompetent and immunocompromised patients. Chest 2006;129:333-40. [PubMed]
- Jarvis JN, Harrison TS. Pulmonary cryptococcosis. Semin Respir Crit Care Med 2008;29:141-50. [PubMed]