How to get the best from robotic thoracic surgery
The interest in robotic-thoracic surgery has grown in the world since this technology was first approved in 2001 by the US Food and Drug Administration (FDA) and in part supported by studies which stressed the equivalence of the oncological results when compared with the “traditional approach” (video assisted thoracic surgery VATS or open surgery). The Robotic surgical system (da Vinci, Intuitive Surgical Inc., Sunnyvale, CA, USA) is to date the only complete surgical platform worldwide available and it is considered the highest technological innovation, thanks to its wrist like manoeuvrability with 7 degrees of freedom, the physiological tremor filtration (6-Hz motion filter) and 3-dimensional imaging. All these features allow the recruitment of surgeons, especially those who perform open surgery, by the claims that the dexterity associated with open approach can be almost replicated by the robotic platform, but without such a steep learning curve than in VATS procedures. In addition, this high technology allows to perform a broad range of complex operations in a safe and comfortable way, maintaining the advantages related to low invasiveness.
Since its first application the robotic system has become widespread in thoracic surgery both for mediastinal pathologies (thymic hyperplasia, thymic malignancy and posterior mediastinal mass) and for lung cancer diseases (1).
The adoption of the robotic surgery in mediastinal pathologies has become popular worldwide thanks to its easier approach and manipulation of narrow anatomic spaces such as the retrosternal area. Moreover, robotic thymectomy seems to lead to higher remission rates of myasthenia gravis compared to thoracoscopic thymectomy, this is probably due to the particular features of the robotic platform that permits an extended thymectomy also in more complex cases (e.g., patients with pectus excavatum, with a previous sternotomy, with high BMI etc.) (2-4).
Furthermore, robotic thymectomy might be considered a safe and effective procedure for thymomas, also in large tumours with equivalent radicality when compared to open procedures yet with lower morbidity and shorter hospital stay (5-7).
Nowadays, little data with an adequate follow-up exists on oncological outcomes, nevertheless, the literature confirms the high rate (almost 90%) of complete R0 resections which is guaranteed both using the robotic system and open approach (8,9).
Another advantageous application of robotic technology has been found when dealing with posterior mediastinal tumours, even though the experience described in the literature is still limited. The uncomfortable posterior mediastinal space can be easily reached by the robotic system with a safe and precise removal of the tumour, this is true also for tumours found in remote areas, guaranteeing less trauma, lower rate of complications, shorter postoperative stay and better aesthetic results (10,11).
Concerning lung cancer, robotic-assisted thoracic surgery (RATS) has been demonstrated feasible, safe and more accurate for vessels isolation (also in the presence of anatomic variations or in case of large tumours which make the lung mobilization difficult), dissection of hilar and mediastinal lymph nodes when compared with thoracoscopic surgery.
Moreover, this technique results in a better quality of life, lower amount of blood loss, lower mortality and morbidity than open approach (12,13).
Notwithstanding the benefits of RATS, given the few thoracic centers with the availability of robotic systems, few reports have been published on long-term oncological outcomes for non-small cell lung cancer (NSCLC) patients treated by robotic lobectomy (14,15).
Using the rate of nodal upstaging as a surrogate of the quality of surgery, several studies have demonstrated that robotic surgery guarantees a similar upstage than thoracotomy and a greater one than in VATS, suggesting that RATS lobectomy reaches an oncological radicality equivalent to the one offered by conventional surgery (12).
Undoubtedly a solid surgical background and a consistent operating activity are mandatory to obtain effective results. Many papers have confirmed that high volume specialized centres and high-volume surgeons have a positive impact on patients’ outcome (16-18).
Moreover, several authors have shown that patients who undergo lobectomy in high-volume centers have a shorter mean length of stay (LOS) and a lower rate of mortality and complications (18-20). In a recent study published by Tchouta et al., a total of 8,253 RATS lobectomies were analysed comparing outcomes, such as LOS, mortality and complications, in very low-volume centers versus high-volume hospitals, finding, through multivariate analysis, that high volume centers were prognostic for decreased mortality and shorter LOS but not for any of the complications. However, performing higher volume RATS without a dedicated program does not seem to guarantee a positive effect on the clinical results. This is probably related to the surgical different experience and the dexterity with robotic system and also could be attributed to the novelty of RATS lobectomy. This procedure has been in use only recently and only in few centers, therefore when compared with Vats lobectomy, used since 1992, it has been associated, evaluating a serious of clinical elements, with an encouraging volume/outcome relationship (20).
One of the most criticized aspects of the robotic platform is its high capital and running cost, therefore only a restrictive number of worldwide centres have the availability of this system. In order to minimize costs and to become competitive, one reasonable strategy is based on high surgical volumes and standardization of the technique that could reduce the surgical procedures time, the number of robotic instruments used and, as described above, the rate of complications and the length of hospitalization (21,22).
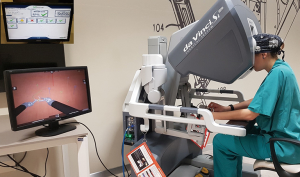
Due to wider community restriction, the possibilities for thoracic trainees to acquire the essential skills in RATS, are limited. Thus an established stepwise strategy for the introduction of robotic surgery into thoracic training program should be strongly considered. Thanks to its technological innovations, the robotic system is equipped with a simulator and dual console (Figure 1). According to our experience the training program consist of 3 steps: the use of the simulator, the observation of cases performed by a skilled surgeon and to perform operations proctored by proficient colleagues as the final step.
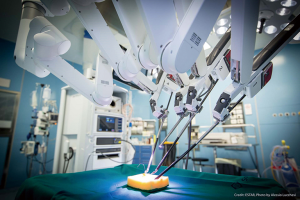
Performing a broad range of exercises, the trainee is able to acquire confidence in the principles of the instruments manipulation. At beginning of the robotic experience, the surgeon must perform repeatedly each exercise to improve his technical performance (Figure 2).
A few numbers of high volume centres are recognised as “observational centres” and they represent a reference point for the whole trainee community to obtain robotic tips and tricks. An important aspect of the learning process is the acquisition of a “standardization of technique” which allows to reduce the learning curve time and consequently the operative time and the number of adverse events (e.g., bleeding and prolonged air leak).
During the early phase of experience, the skilled surgeon can teach and proctor the colleague from the second console, in order to guarantee a safe and effective procedure.
Moreover, in order to give the opportunity to share experience in robotic surgery, three level courses are organized, fitted to different surgical ability (resident or consultant).
The first level consists in basic application of robotic technology where a panel of expert surgeons gives information on the indications of this technique and covers the principles of the Robotic platform application.
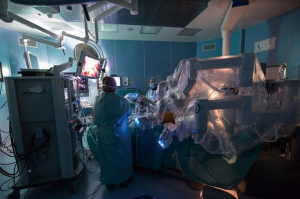
The second level is focalized on using robotic technology in thoracic procedures and on its related clinical aspects. The system preparation, operatory room configuration and intraoperative techniques (such as the application of instruments) are shown to trainees (Figure 3). Animal or cadaver models might exceptionally be used.
The last level of the curse consists in integrated system training, case observation and training at the console finalized to acquire anatomical references and principles of robotic surgical steps. The dual console capability facilitates the proctoring and guarantees the procedure safety allowing the exchange of instruments control between the surgeons.
Nevertheless, it is advisable to begin the learning process with simple procedures like mediastinal lesions removal, and then gradually proceed to more complex surgical procedures, such as major lung resections. The learning curve for robotic surgery is shorter than VATS, it has been demonstrated that 20 RATS lobectomies are sufficient for an experienced thoracic surgeon to become confident enough with this technique (23,24).
To achieve a good level of proficiency the training surgeon should perform a sufficient number of operations autonomously. Consequently a broad range of procedures is necessary for the trainee to acquire dexterity while proctored by a skilled robotic surgeon.
Taking into consideration the technological innovations and the provided benefits both for the patients and for the surgeons of the robotic system, it is clear that its use in thoracic surgery is still evolving as well as its applications and indications. Henceforth, a process focused on the standardization of the technique, cost reduction and trainee tutoring should be widely considered the best way to employ and to take advantage of this highly technological system: this is the key to get the best from robotic thoracic surgery.
Acknowledgements
The Authors thank Teresa Hung Key for the proofreading.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Ruckert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg 2011;141:673-7. [Crossref] [PubMed]
- Rueckert J, Swierzy M, Badakhshi H, et al. Robotic-assisted thymectomy: surgical procedure and results. Thorac Cardiovasc Surg 2015;63:194-200. [Crossref] [PubMed]
- Ricciardi R, Melfi F, Maestri M, et al. Endoscopic thymectomy: a neurologist's perspective. Ann Cardiothorac Surg 2016;5:38-44. [PubMed]
- Keijzers M, Dingemans AM, Blaauwgeers H, et al. 8 years' experience with robotic thymectomy for thymomas. Surg Endosc 2014;28:1202-8. [Crossref] [PubMed]
- Marulli G, Maessen J, Melfi F, et al. Multi-institutional European experience of robotic thymectomy for thymoma. Ann Cardiothorac Surg 2016;5:18-25. [PubMed]
- Kneuertz PJ, Kamel MK, Stiles BM, et al. Robotic Thymectomy Is Feasible for Large Thymomas: A Propensity-Matched Comparison. Ann Thorac Surg 2017;104:1673-8. [Crossref] [PubMed]
- Burt BM, Yao X, Shrager J, et al. Determinants of Complete Resection of Thymoma by Minimally Invasive and Open Thymectomy: Analysis of an International Registry. J Thorac Oncol 2017;12:129-36. [Crossref] [PubMed]
- Friedant AJ, Handorf EA, Su S, et al. Minimally Invasive versus Open Thymectomy for Thymic Malignancies: Systematic Review and Meta-Analysis. J Thorac Oncol 2016;11:30-8. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Operative techniques in robotic thoracic surgery for inferior or posterior mediastinal pathology. J Thorac Cardiovasc Surg 2012;143:1138-43. [Crossref] [PubMed]
- Zirafa CC, Melfi F. Robot-assisted surgery for posterior mediastinal mass. J Thorac Dis 2017;9:4929-31. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Farivar AS, Cerfolio RJ, Vallieres E, et al. Comparing robotic lung resection with thoracotomy and video-assisted thoracoscopic surgery cases entered into the Society of Thoracic Surgeons database. Innovations (Phila) 2014;9:10-5. [Crossref] [PubMed]
- Cerfolio RJ, Ghanim AF, Dylewski M, et al. The long-term survival of robotic lobectomy for non-small cell lung cancer: A multi-institutional study. J Thorac Cardiovasc Surg 2018;155:778-86. [Crossref] [PubMed]
- Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9. [Crossref] [PubMed]
- Bach PB, Cramer LD, Schrag D, et al. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med 2001;345:181-8. [Crossref] [PubMed]
- Cheung MC, Hamilton K, Sherman R, et al. Impact of teaching facility status and high-volume centers on outcomes for lung cancer resection: an examination of 13,469 surgical patients. Ann Surg Oncol 2009;16:3-13. [Crossref] [PubMed]
- Chowdhury MM, Dagash H, Pierro A. A systematic review of the impact of volume of surgery and specialization on patient outcome. Br J Surg 2007;94:145-61. [Crossref] [PubMed]
- Park HS, Detterbeck FC, Boffa DJ, et al. Impact of hospital volume of thoracoscopic lobectomy on primary lung cancer outcomes. Ann Thorac Surg 2012;93:372-9. [Crossref] [PubMed]
- Tchouta LN, Park HS, Boffa DJ, et al. Hospital Volume and Outcomes of Robot-Assisted Lobectomies. Chest 2017;151:329-39. [Crossref] [PubMed]
- Nasir BS, Bryant AS, Minnich DJ, et al. Performing robotic lobectomy and segmentectomy: cost, profitability, and outcomes. Ann Thorac Surg 2014;98:203-8; discussion 8-9. [Crossref] [PubMed]
- Veronesi G, Cerfolio R, Cingolani R, et al. Report on First International Workshop on Robotic Surgery in Thoracic Oncology. Front Oncol 2016;6:214. [Crossref] [PubMed]
- Melfi FM, Mussi A. Robotically assisted lobectomy: learning curve and complications. Thorac Surg Clin 2008;18:289-95. vi-vii. [Crossref] [PubMed]
- Veronesi G, Agoglia BG, Melfi F, et al. Experience with robotic lobectomy for lung cancer. Innovations (Phila) 2011;6:355-60. [Crossref] [PubMed]