Dermatomyositis as an antecedent sign of lung cancer in an eldly patient: a case report
Introduction
Dermatomyositis (DM) is an idiopathic inflammatory myopathy with characteristic cutaneous manifestations including heliotrope rash of the periorbital skin, erythematous scaly plaques on dorsal hands with periungual telangiectasia and photosensitive poikilodermatous eruption. The myopathy is generally symmetrical and slowly progressive during a period of weeks to months affecting mainly the proximal muscles. Besides a role of viral infections or medications as a trigger, clinical reports showed a relationship of the disease with cancer. Sterz reported the first case of DM associated with stomach cancer in 1916 (1) and Rosa et al. reported a case with proximal muscular weakness as only symptom of colon cancer (2). We report a case with a rare and surgically treated DM-associated-lung cancer in an elderly and concerned with the clinical features of this disease.
Case report
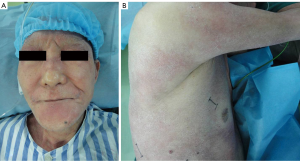
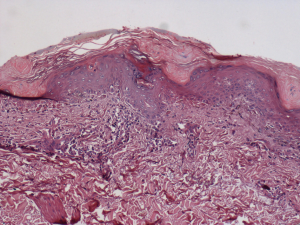
A 73-year-old male was admitted to the local hospital because of the mauve edematous rash on the back merely beginning in June 2012. One month later, the rash, accompanied by mild itching and twinge, became more extensive and diffused to his whole face, trunk, and limbs in a symmetric distribution (Figure 1). At the same time, he developed muscle weakness of upper limbs, especially in his ability to lift his arms above his head and to grip with his hands. A single clinical diagnosis of the DM was confirmed by a biopsy of quadriceps muscle and skin (Figure 2). The epidermis, taken from the bullous region, was thinned, and there was vacuolization at the dermal-epidermal junction. The Alcian blue stain demonstrated normal amounts of the dermal mucin. The periodic acid-Schiff stain was negative for fungi, and the basement membrane was thickened. The muscle biopsy revealed striation atrophy with partial muscle fiber atrophy. Hence, the prednisone, methotrexate and compound glycyrrhizin were administered to him at the local hospital. His weakness improved slightly at the beginning, however, with no additional change in the subsequent days and no other change during the entire prednisone treatment duration. With one month, he developed continuously increasing cough, expectoration and blood-stained sputum. Then, the patient discontinued the corticosteroid medication because he was told by a doctor of the local hospital that the corticosteroids might have induced pulmonary infection. He had smoked one pack of cigarettes per day for the past 30 years and quit smoking for nine years. His family history was unremarkable. He denied any previous medical illnesses and was not taking any medications. The patient, who works as a farmer, denied any possible exposure to occupational hazards or toxic fumes. He had no risk factors for human immunodeficiency disease or other infections.
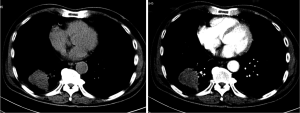
After admission to our hospital in September of 2012, Physical examination showed the malar areas, the bridge of the nose, and the nasolabial folds were covered erythema. The skin was erythematous with blisters and multiple areas of scaling and scars extending over the trunk in a “V” shaped pattern involving the neck, upper back, shoulders and low limbs. His neurologic examination showed intact sensation and reflexes throughout with marked weakness in his extremities and trunk. The Manual Muscle Test (MMT) revealed a Lovett 2 (poor) degree of strength in his limbs. Electromyography (EMG) is characterized by increased irritability with spontaneous fibrillation and sharp waves. Plain and contrast-enhanced chest computed tomography (CT) (Figure 3) revealed a tumor shadow measuring 5.9 cm × 4.3 cm in size in the lower lobe of the right lung. No mediastinal lymph node metastasis or hilar lymph node metastasis were observed. A bronchoscopic examination to elucidate the nature of extrinsic bronchial compression showed no evidence of endobronchial lesion or obstruction. His Pulmonary function tests revealed a reduction in vital capacity and forced expiratory volume. Cardiovascular examination revealed normal performance.
Hematology tests and biochemistry tests showed creatine kinase (CK, 420 U/L; normal value, 25-200 U/L), lactate dehydrogenase (LDH, 756 U/L; normal value, 230-460 U/L), carcinoembryonic antigen (CEA, 300.5 ng/mL; normal value, 0-8 ng/mL), and cyfra 21-1 cancer antigen (CYFRA21-1, 50 ng/mL; normal value, 0-3 ng/mL). Anti-Jo-1 level was negative.
As diagnosis of the mass in the thorax was not established through imaging and bronchoscopic examination, surgery was scheduled. Under general anesthesia with selective intubation, the patient was subjected to the right posterolateral thoracotomy through the seventh intercostal space. Little pleural effusion was observed in the thorax and the adhesions were seen between right lung and chest wall. The tumor located at the right lower lobe had a diameter of about 6.5 cm, and the right lower lobe showed consolidation changes. At last, the resection of the right lower lobe was made. 1 cm2 of the tissue was taken out with a biopsy forceps from the tumor for quick frozen pathology, which was pathologically diagnosed as squamous cell carcinoma. Within one week, normal food intake was renovated and the patient was discharged from the hospital. During consecutive four weeks 20 mg of prednisone was administered in tapering doses. One month later, a chest CT scan showed the residual lobe could finally be fully expanded to an almost standard size and appearance. The rash nearly disappeared and even lower CK (265 U/L), LDH (582 U/L), and CEA (120.2 ng/mL) levels were detected. An MMT revealed a Lovett 4 (good) degree of strength in the lower extremities and a Lovett 3 (fair) degree of strength in the upper extremities. The patient is still alive without relapse of the DM symptoms and is currently receiving follow-up.
Discussion
DM is a disease that can present in individuals of all ages. It has an incidence of 5.5 per million people. The exact mechanism for DM is not known, but it is postulated to have an autoimmune component. In DM, internal organs may be involved and interstitial lung fibrosis can be seen (3). The most important feature in adults with DM, however, is an increased incidence of lung cancer (4).
Age plays an important role in whether DM is associated with lung cancer. The lung cancer frequency in DM patients is higher in elderly patients than younger patients (5). The study of de Souza and Shinjo showed that an 8.6% prevalence of neoplasia in individuals with newly-diagnosed DM (6). The reports of the association between gender and the risk of lung cancer in DM patients were inconsistent. According to the Zhang et al., it was more common in men than in women among patients aged 40 years or above (7). de Souza and Shinjo, however, found that the neoplasias affected predominantly women (6).
The diagnosis of DM is confirmed through clinical history, examination of proximal muscle weakness, skin and muscle biopsy and laboratory criteria include serum muscle enzyme concentrations as well as autoantibody tests. Frequently, LDH, CK, and aminotransferases are elevated from muscle breakdown. Anti-Jo-1 antibody has a high diagnostic specificity, but its positive rate is only 30% of cases (8). Anti-Jo-1 level was negative in our case. Since DM is a systemic disease, it may present with symptoms of internal organs disorder. These sometimes covered the underlying malignancy. In our case, the patient only presented the clinical manifestation of DM, so that he was misdiagnosed as single DM and symptomatic treatment merely without taking the CT in the local hospital.
With regard to DM, treatment is largely based on controlling the likely autoimmune component of the disease. One possible etiology is complement-mediated inflammation at the vascular level; another is a direct cytotoxic effect of lymphocytes on the muscle cells. Initial therapy consists of high-dose steroids. Immunosuppressant and cytotoxic agents are often given early in the course in order to wean off steroids, thereby limiting the toxic effect of chronic steroids. Methotrexate, azathioprine, and mycophenolate mofetil are common agents used in DM. Zang et al. reported that they treated a patient with targeted lung cancer therapy alone (9). The histological type of tumor in the patient was adenocarcinoma and gefitinib was therefore administered to the patient. As a result, an obvious diminution in the primary tumor and a significant decrease in the CEA level were observed. Surgical treatment of DM associated with lung cancer in some cases may lead to a significant decrease in symptoms, or a recovery from DM, also in cases resistant to pharmacological treatment (10). In this case, DM was the paraneoplastic syndrome coexisting with squamous cancer of the right lung. No mediastinal lymph node metastasis or hilar lymph node metastasis were observed from CT and the patient was considered to have the right lower lobectomy.
Our study emphasizes the concept that DM may be associated with lung cancer, and extensive diagnostic work-up to exclude neoplastic lesions should be performed, mainly those with newly diagnosed DM. Among other predictive factors of cancer, DM initiating at advanced age should be considered.
Acknowledgements
We greatly appreciate the assistance of the staff of the Department of Thoracic Surgery, West-China Hospital, Sichuan University, and thank them for their efforts.
Disclosure: The authors declare no conflict of interest.
References
- Sterz G. Polymyositis. Berliner Klinische Wochenschrift 1916;53:489.
- Rosa F, Ferrari M, Buschiazzo A, et al. Myopathy as paraneoplastic syndrome of colon malignancy in an elderly patient. Aging Clin Exp Res 2013;25:221-3. [PubMed]
- Fathi M, Lundberg IE, Tornling G. Pulmonary complications of polymyositis and dermatomyositis. Semin Respir Crit Care Med 2007;28:451-8. [PubMed]
- Przybylski G, Jarzemska A, Czerniak J, et al. A case report of a patient with dermatomyositis as a prodromal sign of lung cancer. Pol Arch Med Wewn 2008;118:143-7. [PubMed]
- Antiochos BB, Brown LA, Li Z, et al. Malignancy is associated with dermatomyositis but not polymyositis in Northern New England, USA. J Rheumatol 2009;36:2704-10. [PubMed]
- de Souza FH, Shinjo SK. Newly diagnosed dermatomyositis in the elderly as predictor of malignancy. Rev Bras Reumatol 2012;52:713-21. [PubMed]
- Zhang W, Jiang SP, Huang L. Dermatomyositis and malignancy: a retrospective study of 115 cases. Eur Rev Med Pharmacol Sci 2009;13:77-80. [PubMed]
- Yamauchi K, Kogashiwa Y, Nagafuji H, et al. Head and neck cancer with dermatomyositis: a report of two clinical cases. Int J Otolaryngol 2010;2010:401825.
- Zang YS, Xiu QY, Fang Z, et al. Case report: dramatic recovery of lung adenocarcinoma-associated dermatomyositis with targeted lung cancer therapy alone. Oncologist 2008;13:79-81. [PubMed]
- Radziszewski K, Litwinowicz L, Borowicz M, et al. Talar: “Zapalenie skórno-mięśniowe “maską” choroby nowotworowej Fizjoterapia Polska 2003;3:184-8.