Clinical evaluation of the utility of a flexible 19-gauge EBUS-TBNA needle
Introduction
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) has succeeded as a highly sensitive, minimally invasive outpatient procedure for mediastinal lymph node staging in lung cancer patients (1). Since many patients who have advanced disease at the time of first presentation are not eligible for surgery, testing for molecular markers using small biopsy samples is frequently attempted. With the ever-increasing number of available markers for testing, specimens obtained with conventional 21- or 22-gauge EBUS-TBNA needles can be insufficient. Therefore, there is still a role for surgical biopsy via mediastinoscopy or video-assisted thoracic surgery when substantial material is required.
EBUS-TBNA samples are now being routinely used for the diagnosis of lung cancer and molecular investigations, including determination of epidermal growth factor receptor (EGFR) mutation status to predict response to treatment and assess prognosis (2-5). Recently, the therapeutic efficacy of immune checkpoint inhibitors in patients with advanced non-small cell lung cancer (NSCLC) has been demonstrated in clinical trials (6,7). Preclinical studies have revealed that therapeutic efficacy is closely associated with the expression of programmed cell death ligand 1 (PD-L1) on lung cancer cells (8). For improved clinical management, evaluation for these immune markers should be performed in lung cancer patients. Adequate samples are indispensable for accurate molecular evaluation of the immunogenic environment. Use of 21 and 22G EBUS-TBNA needles may provide samples which are quantitatively too limited or may require a higher number of needle passes to obtain sufficient sample to evaluate the expression of PD-L1 on lung cancer cells. To improve and increase the options available for sample collection, a new flexible 19-gauge (Flex 19G) EBUS-TBNA needle (Olympus, Redmond, WA, USA) has been developed. This flexible, larger diameter needle potentially provides tissue sufficient for histopathological processing and may facilitate assessment of an increased number of diagnostic and prognostic markers.
Before the final version of the Flex 19G needle was available, we performed product performance evaluations using early versions, and in turn provided feedback to the manufacturer regarding use and performance. Here, we report on the evolution and clinical utility of Flex 19G EBUS-TBNA needles in the assessment of mediastinal lymph nodes and lung tumors.
Methods
Patients
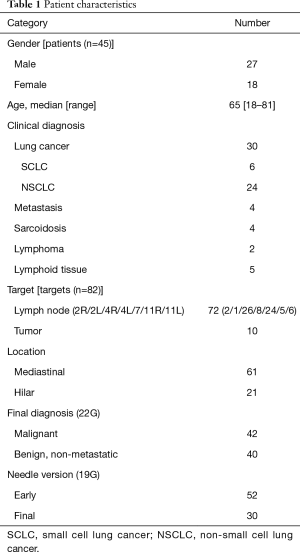
We performed a retrospective analysis of patients who underwent EBUS-TBNA sampling with the standard 22G needle and the Flex 19G needle at Toronto General Hospital from October 2014 to January 2017 (Table 1). All patients underwent chest CT scanning prior to EBUS-TBNA. The definition of each lymph node station was based on the 7th edition of the TNM classification for lung cancer (9). The original Flex 19G needle evaluation was approved by the Research Ethics Board of University Health Network (approval no. 16-5341).
Full table
EBUS and EBUS-TBNA needle
EBUS-TBNA was performed under local anesthesia with conscious sedation using midazolam and fentanyl. A convex probe endobronchial ultrasound (CP-EBUS, BF-UC180F, Olympus, Tokyo, Japan) was used for EBUS-TBNA. The ultrasound image was processed with a universal endoscopic ultrasound scanner (EU-ME1; Olympus, Tokyo, Japan). EBUS-TBNA procedures were performed in the same manner as previously described (10). We initially advanced the ViziShot 22G (Olympus, Tokyo, Japan) EBUS-TBNA needle toward the target site and collected cytological samples. In the same setting, the Flex 19G needle was used for tissue sampling at the same targets (Figure 1A). The Flex 19G needles included an early version (Oct/2014–Sep/2015) and an improved final version (May/2016–Jan/2017). Compared to the prototype version, this improved needle was stiffened for smoother tracheal/bronchial wall puncture, among other design enhancements.

Pathological evaluation
Samples obtained with 22G needles were processed in methanol-containing fixative (CytoLyt; Cytyc Corporation; Marlborough, MA) and promptly delivered to the cytology laboratory for preparation of cell blocks. Rapid on-site cytological evaluation was not performed routinely. Samples obtained with Flex 19G needles were expelled on filter paper, fixed in formalin and submitted separately for histological processing (Figure 1B). Specimen cores were submitted for histopathology only if the endoscopist felt they were adequate. Specimen volume was measured by the pathology team using calipers. Cellularity was defined as the numbers of cells per slide. Samples processed for cytological and histopathological evaluation were independently reviewed by subspecialty cytopathologists and surgical pathologists, respectively.
Data analysis
In this retrospective study, the success rate of obtaining cores with the 19G needle, volume of the specimen, and safety were assessed. The volume evaluation of targets was calculated based on the measurement data by ultrasound imaging as follows.
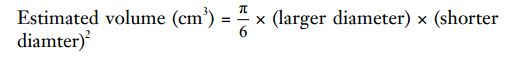
The number of punctures (also referred to in our paper as passes) with each needle were documented. The diagnostic yield and other interval measurements of both groups were compared using a χ2 test, Mann-Whitney U test, or paired t-test. All tests were two-sided and P values of <0.05 were considered statistically significant. Data was analyzed with the statistical Standard Package Source Solution Software (SPSS standard version 24.0; SPSS Inc., Chicago, Illinois).
Results
A total of 72 lymph nodes (mediastinal 61, hilar 11) and 10 hilar tumors in 45 patients were sampled. All procedures were performed without complication, and the overall diagnostic yield from cytology by 22G needle was 100% (Table 1).
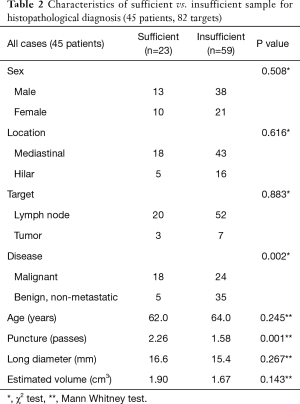
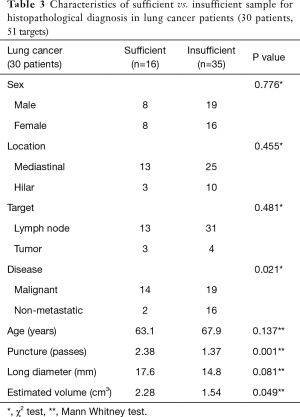
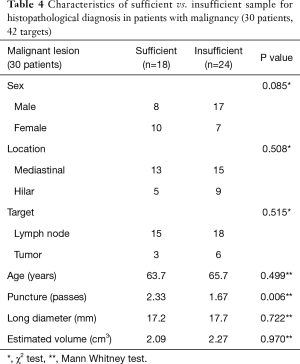
The use of the 19G needle resulted in 28 solid core samples (34.1%) for histological analysis, with 23 out of 28 samples (82.1%) sufficient for histopathological diagnosis (as determined by sample cellularity and condition). In the remaining 54 samples, operators chose not to submit the specimen to pathology due to their appearance on filtered paper (sample too small, sample was mostly blood clot, etc.). The overall success rate to obtain enough tissue for diagnosis was 28.0% (Tables 2-4). The mean volume of the 23 sufficient samples was 21.5 mm3. Compared to the 59 insufficient cases, we could obtain diagnostic cores more often in malignant lesions (78.2% vs. 40.7%, P=0.002) and with more passes (2.26 vs. 1.58, P=0.001) (Tables 2-4). In the subgroup analysis of patients with lung cancer (30 patients, 51 targets), sufficient cores were obtained in 31.4%. Sufficient cores for diagnosis were obtained with more passes (2.38 vs. 1.37, P=0.001), larger targets (2.28 vs. 1.54 cm3, P=0.049), and malignant targets (87.5% vs. 54.3%, P=0.021). Subgroup analysis of malignant targets diagnosed by cytology suggests that malignant targets may be confounder for target size and sample sufficiency. In malignant targets, the only factor that influenced the success rate was number of punctures (2.33 vs. 1.67, P=0.006) (Tables 2-4).
Full table
Full table
Full table
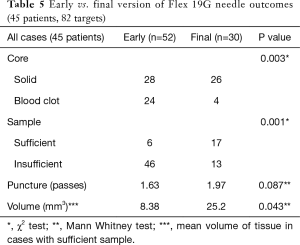
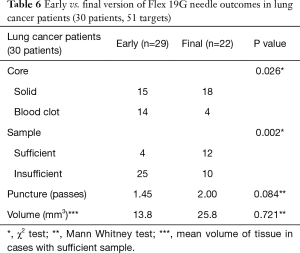
A subgroup analysis was performed to compare the prototype Flex 19G needle with the final commercial version, to assess whether future evaluation could result in improved outcomes. The early version Flex 19G needle, used in 20 patients and 52 targets, provided samples that were on average 8.38 mm3 in size. Of them, 28 (53.8%) were cores and 24 (46.2%) were blood clot samples. Only 6 diagnostic cores (21.4%) were obtained. On the other hand, the final version of the Flex 19G needle, used in 20 patients and 30 targets, provided samples that were an average 25.2 mm3 in size. Overall 26 samples (86.7%) were cores and only 4 (13.3%) were blood clot samples. Among cores, 17 were diagnostic (65.4%) on histologic assessment. When comparing final versus early versions, these differences were statistically significant. The average number of puncture passes was 1.63 using the early version versus 1.97 using the final version. This was not statistically significant (Tables 5-7). When evaluating the subgroups of lung cancer patients and malignant lesions, the final version of the Flex 19G needle had a significantly increased frequency of cores. The final version of Flex 19G also appears to possibly provide a larger volumetric sample, though this was non-significant (Tables 5-7).
Full table
Full table

Full table
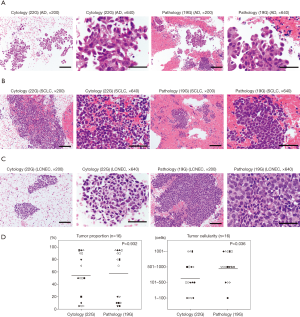
Figure 2A,B,C provide representative pictures of histopathological specimens obtained with the Flex 19G needle and cytological specimens obtained with the 22G needle. In the group evaluated with the final Flex 19G needle type, 16 targets had lung cancer cells. No statistical difference of tumor cell proportion between cytology and histology samples was found among these specimens. However, tumor cellularity was significantly higher in the histopathological samples from Flex 19G (P=0.036) (Figure 2D, Table S1). These histopathology samples also tended to be more cohesive, with maintained tissue architecture and a more visible stroma/interstitial component.
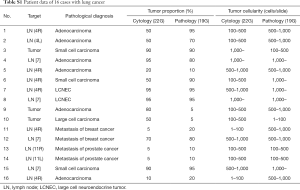
Full table
Discussion
Non-EBUS 19G needles have been used in conventional TBNA by others. These groups have reported the utility and efficacy of a hybrid method employing a standard 22G EBUS-TBNA needle followed by 19G TBNA needle to sample mediastinal lesions, with promising results for cyto- and histopathological evaluation (11,12). Recently, a flexible 19G fine aspiration needle has been developed for gastrointestinal endoscopy and endoscopic ultrasound-guided rendezvous procedures (13). We have previously reported the safety and feasibility of a first-generation flexible 19G EBUS-TBNA needle (14). Over time, several versions of this needle became available for clinical evaluation as the manufacturer was refining the product for commercial launch. This manuscript provides information on the overall performance of the Flex 19G needle, with promising preliminary data that the newer (final) version may outperform an older (early) version. As the Flex 19G EBUS-TBNA needle is now commercially available, additional investigations should be conducted not only to confirm its safety but also to evaluate its utility for conditions such as cancer, lymphoma, and sarcoidosis.
The sensitivity and specificity of cytological samples collected with EBUS-TBNA have improved with greater bronchoscopist and cytopathologist experience (15,16). Our understanding of specific biologic factors that affect the response to chemotherapy and immunotherapy in lung cancers is evolving. While the debate regarding the adequacy of cytological versus histological samples for molecular diagnosis is ongoing (17), some biological factors rely on histopathological assessment, like PD-L1 expression on tumor cells for immune checkpoint blockade treatment (8,18). In terms of PD-L1 evaluation, the concordance between cytological samples and core tissue is not confirmed (19). However, we predict 19G needle transbronchial lymph node biopsy could perform similar to solid tissue biopsy, with potential to perform PD-L1, EGFR and anaplastic lymphoma kinase (ALK) testing (20). This would ideally avoid the need for repeated procedures for said molecular testing. Beyond lung cancer, other malignant diseases such as lymphoma require sufficient tumor cellularity to determine World Health Organization classification. EBUS-TBNA with the Flex 19G needle has the ability to obtain larger samples, which may allow for detailed immunohistochemical analysis of lymph node metastases and the tumor itself. This may help provide additional prognostic information to guide disease-specific therapy. Our data would support that the Flex 19G needle can provide a significant improvement in cellularity and tissue volume to realize these potential benefits.
Although EBUS-TBNA is a safe and cost effective diagnostic procedure for staging lung cancer, the low success rate of obtaining tissue (core) samples has been identified as a disadvantage of small diameter needles (21). The flexible 19G EBUS-TBNA needle has the potential to provide adequate larger samples. Combining cytological and histological evaluation could provide a more accurate diagnosis, such as improved subclassification of NSCLC or diagnosis of lymphoma. Therefore, advanced EBUS-TBNA techniques with Flex 19G needle may provide an advantage with regards to minimal invasiveness, diagnostic yield, and medical expenditure (as it relates to the need for further diagnostic procedures).
As this retrospective study was not conducted to directly compare the diagnostic yield of 19G and 22G needles, our results do not suggest that a 19G needle should be used instead of a 22G needle. Judicious use of different gauge needles depending on the characteristics of the target and the feedback given by the on-site pathologist is recommended. Highly cellular and vascular targets may require a smaller needle, whereas Flex 19G may be advantageous when more tissue is desired or underlying tissue architecture, such as fibrosis, precludes adequate sampling with smaller needles.
Our study has several limitations. It is a retrospective study at a single institution, with procedures performed mainly by a single endoscopist and therefore subject to the endoscopist’s experience and biases, limiting generalizability. Also, the study was done at different times with different versions of the product and used a limited (less than recommended) number of sampling passes because sampling was done first with a 22G needle. Another limitation is that total numbers of enrolled patients and targets are too small to confirm clinical advantage of Flex 19G EBUS-TBNA needle. Finally, the feasibility of cytological diagnosis by 19G EBUS-TBNA needle was not evaluated in this study as it was done with the 22G needle.
Conclusions
The final version of the Flex 19G EBUS-TBNA needle evaluated in this retrospective study can safely provide larger volumetric and core tissue samples that are appropriate for histopathological diagnosis in cancer and malignant lesions. The Flex 19G should be used in selected cases where greater tissue acquisition is required, and can provide adequate tissue from malignant lesions, which is important for molecular analysis. Further study of the Flex 19G needle is warranted based on these promising early results.
Acknowledgements
The authors are grateful to Ms. Kimberley Hudson, Ms. Patrycja Bauer, Ms. Judy McConnell, and Ms. Alexandria Grindlay (Toronto General Hospital) for sample collection and laboratory management.
Footnote
Conflicts of Interest: K Yasufuku and K Czarnecka-Kujwa are consultants for Olympus America Inc. K Yasufuku has received educational and research grants from Olympus Corporation.
Ethical Statement: The original Flex 19G needle evaluation was approved by the Research Ethics Board of University Health Network (approval no. 16-5341).
References
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Nakajima T, Yasufuku K, Suzuki M, et al. Assessment of epidermal growth factor receptor mutation by endobronchial ultrasound-guided transbronchial needle aspiration. Chest 2007;132:597-602. [Crossref] [PubMed]
- Mohamed S, Yasufuku K, Nakajima T, et al. Analysis of cell cycle-related proteins in mediastinal lymph nodes of patients with N2-NSCLC obtained by EBUS-TBNA: relevance to chemotherapy response. Thorax 2008;63:642-7. [Crossref] [PubMed]
- Nakajima T, Yasufuku K, Suzuki M, et al. Assessment of chemosensitivity-related aberrant methylation of nonsmall cell lung cancer by EBUS-TBNA. J Bronchology Interv Pulmonol 2009;16:10-4. [Crossref] [PubMed]
- Sakairi Y, Nakajima T, Yasufuku K, et al. EML4-ALK fusion gene assessment using metastatic lymph node samples obtained by endobronchial ultrasound-guided transbronchial needle aspiration. Clin Cancer Res 2010;16:4938-45. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:504-35. [Crossref] [PubMed]
- Asamura H, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:1675-84.
- Tyan C, Patel P, Czarnecka K, et al. Flexible 19-gauge endobronchial ultrasound-guided transbronchial needle aspiration needle: first experience. Respiration 2017;94:52-7. [Crossref] [PubMed]
- Herth FJ, Morgan RK, Eberhardt R, et al. Endobronchial ultrasound-guided miniforceps biopsy in the biopsy of subcarinal masses in patients with low likelihood of non-small cell lung cancer. Ann Thorac Surg 2008;85:1874-8. [Crossref] [PubMed]
- Ben S, Akulian J, Wang KP. Endobronchial ultrasound transbronchial needle aspiration: a hybrid method. J Thorac Dis 2015;7:S287-91. [PubMed]
- Tang Z, Igbinomwanhia E, Elhanafi S, et al. Endoscopic ultrasound guided rendezvous drainage of biliary obstruction using a new flexible 19-gauge fine needle aspiration needle. Diagn Ther Endosc 2016;2016:3125962. [PubMed]
- Klimontov VV, Tyan NV, Orlov NB, et al. Association of serum levels and gene polymorphism of vascular endothelium growth factor with ischemic heart disease in type 2 diabetic patients. Kardiologiia 2017;57:17-22. [PubMed]
- Stoll LM, Yung RC, Clark DP, et al. Cytology of endobronchial ultrasound-guided transbronchial needle aspiration versus conventional transbronchial needle aspiration. Cancer Cytopathol 2010;118:278-86. [Crossref] [PubMed]
- Yasufuku K, Chiyo M, Sekine Y, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest 2004;126:122-8. [Crossref] [PubMed]
- Kerr KM, Bubendorf L, Edelman MJ, et al. Second ESMO consensus conference on lung cancer: pathology and molecular biomarkers for non-small-cell lung cancer. Ann Oncol 2014;25:1681-90. [Crossref] [PubMed]
- Iafolla MAJ, Juergens RA. Update on Programmed Death-1 and Programmed Death-Ligand 1 Inhibition in the Treatment of Advanced or Metastatic Non-Small Cell Lung Cancer. Front Oncol 2017;7:67. [Crossref] [PubMed]
- Heymann JJ, Bulman WA, Swinarski D, et al. PD-L1 expression in non-small cell lung carcinoma: Comparison among cytology, small biopsy, and surgical resection specimens. Cancer Cytopathol 2017;125:896-907. [Crossref] [PubMed]
- Herath S, Cooper WA. The novel 19G endobronchial USS (EBUS) needle samples processed as tissue "core biopsies" facilitate PD-L1 and other biomarker testing in lung cancer specimens: case report and the view point from the Respiratory Physician and the Pathologist. Respirol Case Rep 2017;5:e00271. [Crossref] [PubMed]
- Czarnecka-Kujawa K, Rochau U, Siebert U, et al. Cost-effectiveness of mediastinal lymph node staging in non-small cell lung cancer. J Thorac Cardiovasc Surg 2017;153:1567-78. [Crossref] [PubMed]


