Efficacy of adjuvant chemotherapy for completely resected stage IB non-small cell lung cancer: a retrospective study
Introduction
Lung cancer is the most-common cause of loss of productivity and life, as it leads to premature cancer-related mortality (1-3). Furthermore, most cases of lung cancer are detected at an old age, resulting in poor prognosis (4). Early detection may allow treatment at early stages and improve the prognosis of lung cancer. Although some randomized clinical trials showed unsuccessful results of low-dose computed tomography for lung-cancer screening (5), recent studies have revealed that it can detect lung cancer early and thereby reduce mortality (6,7). Moreover, computer-aided detection systems have reduced the errors and false-negative rates, improved detection rate, and diagnosed lung cancer more efficiently (8,9). Therefore, early detection of lung cancer can be frequently conducted, allowing management of early stage lung cancer. However, thus far, there is no consensus on the post-operative management of stage IB non-small cell lung cancer (NSCLC).
On the basis of the results of a previous meta-analysis (10), an international trial was conducted to determine the effects of adjuvant chemotherapy in lung cancer. The trial showed that cisplatin-based adjuvant chemotherapy improves survival of NSCLC in patients who underwent complete resection surgery (11). Following this trial, several studies have been conducted on stage IB NSCLC: some reported positive effects of adjuvant chemotherapy (12,13), whereas others reported negative effects (11,14,15). Strauss et al. showed that adjuvant chemotherapy had a positive effect on patients with stage IB tumors measuring >4 cm (16). In addition, the recent National Comprehensive Cancer Network (NCCN) guidelines stated that adjuvant chemotherapy can be used for patients with stage IB NSCLC having high-risk factors such as poorly differentiated tumor, vascular invasion, wedge resection, tumor size >4 cm, visceral pleural invasion, and incomplete lymph node (LN) sampling (17). However, the evidence to support this guideline is insufficient.
Therefore, this study aimed to identify the predictive factors for prognosis of stage IB NSCLC and to determine the efficacy of adjuvant chemotherapy on recurrence and survival in patients with stage IB NSCLC, especially in high-risk patients.
Methods
Study population
We analyzed 1,316 patients who underwent thoracic surgery at Gangnam Severance Hospital from Jan 2005 to Dec 2014. A total of 556 patients were diagnosed with lung cancer, of which 90 patients with stage IB NSCLC were enrolled. All the patients underwent one of the following definite complete resection surgeries: wedge resection, lobectomy, or pneumonectomy with systematic LN dissection. Exclusion criteria were as follows: serious comorbidity that could influence survival such as other cancers, synchronous lung cancer (two or more histologically distinct simultaneously detected malignancies), and small cell lung cancer (SCLC). After exclusion of one patient with synchronous lung cancer, 89 patients were finally recruited in this study. The requirement of patients’ informed consent was waived owing to the retrospective nature of the study by the Institutional Review Board (IRB) of Gangnam Severance Hospital (number: 3-2016-0276).
Adjuvant chemotherapy
We defined adjuvant chemotherapy as post-operative chemotherapy involving the use of platinum-based agents; the therapy regimen was selected by respective clinicians. Most regimens included cisplatin (75 mg/m2), carboplatin combined with vinorelbine (25 mg/m2), or paclitaxel. Usually, 4 cycles of chemotherapy were performed using these regimens, and there was no definite indication of adjuvant therapy. Although the follow-up schedule varied, usually, chest computed tomography was performed every 3–6 months for the first 2 years, followed by once every 6–12 years. If recurrence was suspected, integrated positron emission tomography-computed tomography was performed.
Pathologic analysis
For histopathologic analysis, two pathology specialists reviewed all the samples of patients, independently. They had no knowledge of those patients’ clinical outcomes. A decision was made on the basis of 2015 World Health Organization classification (18). If they have different opinions, they met, discussed, and reached a final diagnosis by consensus. Hematoxylin and eosin staining was performed for all samples, and additional staining was performed, when needed, according to the decisions of the pathology specialist. Lymphovascular invasion (LVI) was defined as the presence of tumor cells or emboli in the lymphatic or blood vessel lumen (19). Visceral pleural invasion (VPI) was defined as the presence of any distortion of the pleural elastic layer caused by malignant cells (20).
Definition of terms
As per the NCCN guidelines, high-risk patients were defined as those with poorly differentiated tumors, wedge resection, vascular invasion, tumor size >4 cm, VPI, and incomplete LN sampling. However, as only two patients underwent wedge resection and none underwent incomplete LN sampling, these two factors could not be included in this study. Therefore, after elimination of these factors, we defined high-risk patients as those with poorly differentiated tumors, vascular invasion, tumor size >4 cm, and VPI. Disease-free survival (DFS) was defined as the period between the date of resection surgery and the date of recurrence, death, or the last date on which the patient was known to be alive. Overall survival (OS) was defined as the period between the surgery date and the death or the last date on which the patient was known to be alive.
Statistical analysis
The t-test and chi-square test were used to compare continuous variables and categorical variables between the two groups, respectively. All univariate and multivariate analyses for DFS and OS were performed using the Cox regression models. However, univariate analysis for survival curve was performed using the Kaplan-Meier method and the log-rank test. For the multivariate analysis, we used all the parameters included in this study as covariates, except the pathologic type of NSCLC (because the statistical value was immeasurable in univariate analysis) and predominant type (because of many missing values, as it was applicable only to the adenocarcinoma type). All P values <0.05 were considered statistically significant.
Results
Clinical characteristics of subjects
Among the 89 subjects, 27 underwent adjuvant chemotherapy. The adjuvant chemotherapy group had more elderly patients (>65 years) (40.7%) than the control group who did not undergo adjuvant chemotherapy (64.5%, P=0.037). The control group had more patients with squamous cell lung cancer (40.3%), whereas the adjuvant chemotherapy group more patients with adenocarcinoma lung cancer (74.1%, P=0.002). There was no significant difference in the sex, operation type, tumor size, predominant type, differentiation, VPI, LVI, and perineural invasion between the control and adjuvant chemotherapy groups (Table 1).
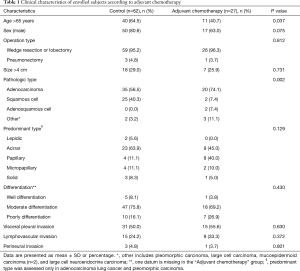
Full table
Univariate analyses for DFS and OS
We tried to find any significant factors for survival using Cox-regression. Age, sex, operation type, size, pathologic type, predominant type, LVI, perineural invasion, and adjuvant chemotherapy was not significant predictive factor for DFS and OS. However, among all the factors analyzed, only VPI was a significant risk factor for DFS [hazard ratio (HR): 7.051; 95% confidence interval (CI): 1.570–31.659; P=0.011) and OS (HR: 8.289; 95% CI: 1.036–66.307; P=0.046) (Table 2).
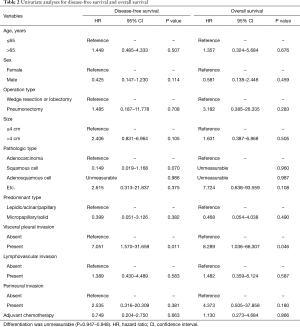
Full table
Effect of adjuvant chemotherapy on DFS and OS according to the adjuvant chemotherapy
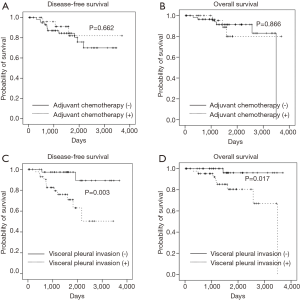
Kaplan-Meier survival analysis did not show any significant difference in DFS (P=0.662) and OS (P=0.866) between the two groups (Figure 1). We tried various combinations of factors to determine whether adjuvant chemotherapy is a significant predictor of survival. However, combinations of age, pathologic type, VPI, and etc., did not show any significant effect of adjuvant chemotherapy on DFS and OS (data are not shown).
Some patients were classified as high-risk patients (19.1% with poorly differentiated tumor, 27.0% with LVI, 28.1% with tumor size >4 cm, and 51.7% with VPI) as per the NCCN guideline definitions. However, even in these patients, adjuvant chemotherapy was not a significant factor for DFS and OS (Table 3).
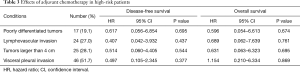
Full table
Effect of VPI on DFS and OS according to the VPI
Kaplan-Meier survival analysis showed that VPI was a significant factor for DFS (P=0.003) and OS (P=0.017) (Figure 1). Multivariate analysis of VPI for survival showed that after adjustment for age, VPI was a significant risk factor for DFS (HR: 7.528; 95% CI: 1.664–34.059; P=0.009) and OS (HR: 8.748; 95% CI: 1.087–70.387; P=0.041). After adjustment for age and sex, VPI was still a significant risk factor for DFS and OS. However, after adjustment for other covariates including pathologic type, operation type, tumor size, LVI, perineural invasion, and adjuvant chemotherapy, VPI was a significant risk factor for DFS, but not for OS (Table 4).
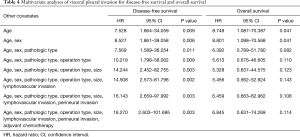
Full table
Discussion
Although adjuvant chemotherapy is routinely performed for patients with stage II–III NSCLC, it has a negative effect on stage IA NSCLC due to its chemotoxicity and immunosuppressive effects (21). The effects of adjuvant chemotherapy on completely resected stage IB NSCLC are controversial: Some studies have shown beneficial effects of adjuvant chemotherapy (13,22,23), whereas others have shown non-significant effects on OS (12,16,24). Further, according to the NCCN guidelines, patients with completely resected stage IB NSCLC may not require adjuvant therapy, but high-risk patients may require chemotherapy. Moreover, in the literature, there are no absolute indications for adjuvant chemotherapy of completely resected stage IB NSCLC. With an aim to determine the effects of adjuvant chemotherapy on stage IB NSCLC, the present study showed that adjuvant chemotherapy does not have any significant benefits for stage IB NSCLC, even for high-risk patients.
The NCCN guidelines recommend that poorly differentiated tumors, LVI, wedge resection, tumor size >4 cm, VPI, and incomplete LN sampling are high-risk factors and can be considered when determining treatment with adjuvant chemotherapy. However, these factors have not proven to be independent factors in well-designed studies. Many clinicians decide to administer adjuvant chemotherapy on the basis of their experience and other recommendations for managing completely resected stage IB NSCLC. However, we found that these above factors are neither powerful for OS prognosis nor important for deciding whether to administer adjuvant chemotherapy. Therefore, adjuvant chemotherapy should be carefully considered for patients with completely resected stage IB NSCLC, and reliable detailed criteria should be established for selection of subjects.
Our study showed that VPI is a strong predictive factor for DFS and OS. In the 1970s, Brewer et al. reported that patients with VPI had a significantly poor prognosis (25); consistent with their findings, many studies confirmed that VPI is an independent factor for poor prognosis of NSCLC (26,27). Furthermore, recent studies showed that VPI is an adverse prognostic factor for early stage NSCLC (28,29). This could be because tumors with VPI are likely to break through the visceral pleura and lead to pleural intraluminal metastasis. Our current results are consistent with those of the above-mentioned studies. However, in high-risk patients with VPI, adjuvant chemotherapy did not significantly affect the prognosis of NSCLC. Therefore, we believe that VPI is a significant risk factor for prognosis, but not a decisive factor for adjuvant chemotherapy.
Adjuvant chemotherapy is frequently performed for young patients and patients with adenocarcinoma type rather than old patients and those with squamous cell type in this study. This might be because the toxicity of adjuvant chemotherapy is higher at an old age than at a young age. Furthermore, the relatively good prognosis of squamous cell lung cancer might deter clinicians from administering adjuvant chemotherapy (30). However, the present study reported that tumor size, differentiation, LVI, and VPI, which are defined as high-risk factors in the NCCN guidelines, need not be considered when making a decision about adjuvant chemotherapy. Although many researchers rely on their own experience and other recommendations with insufficient evidence to make a decision about adjuvant chemotherapy, it is important to note that our data are sourced from only one institute (with five pulmonologists); therefore, our results should be carefully considered and applied.
Despite our important findings, this study had a few limitations. First, it included a small number of patients and short period, which might have yielded suboptimal results. Especially, subgroup analysis cannot have powerful credit. Then, efficacy of adjuvant chemotherapy should be carefully assessed. Second, we included some patients who had recently undergone surgery. Such inclusion could result in confounding findings, with a high censor rate in statistical analysis. Third, only two patients in our cohort underwent wedge resection and none of the patients underwent incomplete LN sampling. Therefore, we could not determine whether these factors are high-risk factors that should be considered when making a decision about adjuvant chemotherapy. Fourth, physicians decided adjuvant chemotherapy; and this bias might lead insignificant results of adjuvant chemotherapy. Last, we did not determine the amount of VPI, quantitatively. Sub-group analysis between focal invasion and extensive invasion will be interesting.
In conclusion, this study suggested that adjuvant chemotherapy may not be significantly effective for the treatment of patients with stage IB NSCLC, even for high-risk patients. In addition, VPI is a strong prognostic factor for survival. Further long-term and multi-center prospective studies are required to confirm our findings.
Acknowledgements
This study was financially supported by Boryung Pharmaceuticals, Ltd.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board (IRB) of Gangnam Severance Hospital (number: 3-2016-0276). The requirement of patients’ informed consent was waived owing to the retrospective nature of the study.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Murray CJ, Atkinson C, Bhalla K, et al. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA 2013;310:591-608. [Crossref] [PubMed]
- Hanly P, Soerjomataram I, Sharp L. Measuring the societal burden of cancer: the cost of lost productivity due to premature cancer-related mortality in Europe. Int J Cancer 2015;136:E136-45. [Crossref] [PubMed]
- Chen VW, Ruiz BA, Hsieh MC, et al. Analysis of stage and clinical/prognostic factors for lung cancer from SEER registries: AJCC staging and collaborative stage data collection system. Cancer 2014;120 Suppl 23:3781-92. [Crossref] [PubMed]
- Infante M, Cavuto S, Lutman FR, et al. Long-Term Follow-up Results of the DANTE Trial, a Randomized Study of Lung Cancer Screening with Spiral Computed Tomography. Am J Respir Crit Care Med 2015;191:1166-75. [Crossref] [PubMed]
- Horeweg N, Scholten ET, de Jong PA, et al. Detection of lung cancer through low-dose CT screening (NELSON): a prespecified analysis of screening test performance and interval cancers. Lancet Oncol 2014;15:1342-50. [Crossref] [PubMed]
- National Lung Screening Trial Research T, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Fraioli F, Bertoletti L, Napoli A, et al. Computer-aided detection (CAD) in lung cancer screening at chest MDCT: ROC analysis of CAD versus radiologist performance. J Thorac Imaging 2007;22:241-6. [Crossref] [PubMed]
- Zhao Y, de Bock GH, Vliegenthart R, et al. Performance of computer-aided detection of pulmonary nodules in low-dose CT: comparison with double reading by nodule volume. Eur Radiol 2012;22:2076-84. [Crossref] [PubMed]
- Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ 1995;311:899-909. [Crossref] [PubMed]
- Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351-60. [Crossref] [PubMed]
- Butts CA, Ding K, Seymour L, et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: updated survival analysis of JBR-10. J Clin Oncol 2010;28:29-34. [Crossref] [PubMed]
- Jang HJ, Cho S, Kim K, et al. Effect of Adjuvant Chemotherapy after Complete Resection for Pathologic Stage IB Lung Adenocarcinoma in High-Risk Patients as Defined by a New Recurrence Risk Scoring Model. Cancer Res Treat 2017;49:898-905. [Crossref] [PubMed]
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7:719-27. [Crossref] [PubMed]
- Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 2005;352:2589-97. [Crossref] [PubMed]
- Strauss GM, Herndon JE 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 2008;26:5043-51. [Crossref] [PubMed]
- Carlson RW, Larsen JK, McClure J, et al. International adaptations of NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2014;12:643-8. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- O'Donnell RK, Feldman M, Mick R, et al. Immunohistochemical method identifies lymphovascular invasion in a majority of oral squamous cell carcinomas and discriminates between blood and lymphatic vessel invasion. J Histochem Cytochem 2008;56:803-10. [Crossref] [PubMed]
- David E, Thall PF, Kalhor N, et al. Visceral pleural invasion is not predictive of survival in patients with lung cancer and smaller tumor size. Ann Thorac Surg 2013;95:1872-7; discussion 1877.
- Ramnath N, Dilling TJ, Harris LJ, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e314S-e340S.
- Kato H, Ichinose Y, Ohta M, et al. A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med 2004;350:1713-21. [Crossref] [PubMed]
- Roselli M, Mariotti S, Ferroni P, et al. Postsurgical chemotherapy in stage IB nonsmall cell lung cancer: Long-term survival in a randomized study. Int J Cancer 2006;119:955-60. [Crossref] [PubMed]
- Park JH, Lee CT, Lee HW, et al. Postoperative adjuvant chemotherapy for stage I non-small cell lung cancer. Eur J Cardiothorac Surg 2005;27:1086-91. [Crossref] [PubMed]
- Brewer LA. Patterns of survival in lung cancer. Chest 1977;71:644-50. [Crossref] [PubMed]
- Shimizu K, Yoshida J, Nagai K, et al. Visceral pleural invasion classification in non-small cell lung cancer: a proposal on the basis of outcome assessment. J Thorac Cardiovasc Surg 2004;127:1574-8. [Crossref] [PubMed]
- Manac'h D, Riquet M, Medioni J, et al. Visceral pleura invasion by non-small cell lung cancer: an underrated bad prognostic factor. Ann Thorac Surg 2001;71:1088-93. [Crossref] [PubMed]
- Tian D, Pei Y, Zheng Q, et al. Effect of visceral pleural invasion on the prognosis of patients with lymph node negative non-small cell lung cancer. Thorac Cancer 2017;8:97-105. [Crossref] [PubMed]
- Liu QX, Deng XF, Zhou D, et al. Visceral pleural invasion impacts the prognosis of non-small cell lung cancer: A meta-analysis. Eur J Surg Oncol 2016;42:1707-13. [Crossref] [PubMed]
- Mountain CF, Lukeman JM, Hammar SP, et al. Lung cancer classification: the relationship of disease extent and cell type to survival in a clinical trials population. J Surg Oncol 1987;35:147-56. [Crossref] [PubMed]


