Contemporary applications of intra-aortic balloon counterpulsation for cardiogenic shock: a “real world” experience
Introduction
The intra-aortic balloon pump (IABP) is the most widely used mechanical circulatory support (MCS), with an implantation rate in USA of around 50,000 per year (1).
However, recent studies (2,3) have challenged the role of IABP support in cardiogenic shock (CS) following ST-elevation myocardial infarction (STEMI) and, independently from these results, on the basis of previous registries, the use of IABP support in CS has been downgraded in the most recent guidelines of American College of Cardiology/American Heart Association (ACC/AHA) (4) and European Society of Cardiology (ESC) (5).
In addition, definitive evidence in other fields of application of IABP, like as peri-procedural support in high-risk percutaneous coronary intervention (PCI) (6) and coronary artery bypass grafting (CABG) (7), is still inconclusive.
The present study aimed to analyze the current clinical practice and results of IABP use in a tertiary care shock center, focusing the analysis on the subset of CS patients, describing their mortality rate, length of intensive care unit (ICU)-stay and identifying the factors associated with in-hospital mortality at the time of IABP implantation. Complications related to the use of the device were also analyzed.
Methods
The study protocol was approved by the Institutional Review Board of the San Raffaele Scientific Institute, Milan, Italy, and was performed in accordance with ethical principles of the Helsinki Declaration. Collection and merging of data were made in agreement with Italian and European data protection laws.
A prospective cohort study was conducted from November 2012 to December 2013, including all patients who underwent IABP support during their hospitalization in the cardiac surgery ICU of San Raffaele Hospital, Milan, a 14-bed ICU serving all critically ill patients from cardiac surgery, catheterization laboratory (cath-lab), electrophysiology department and emergency room.
Primary outcome of the study was to compare medical CS patients (not having had cardiac surgery) and cardiac surgical patients undergoing IABP support in terms of mortality and length of ICU stay.
Secondary outcomes were: to compare characteristics, echocardiographic findings and complications of IABP support in medical CS shock versus cardiac surgical patients; to identify complications related with length of IABP support, to identify factors associated with mortality; to identify an inotropic score threshold that could discriminate on survival in this population.
Primary outcomes were hospital mortality and length of ICU stay.
Secondary outcomes were: to identify factors associated with mortality and ICU stay and complications of IABP support.
Data were prospectively collected using Filemaker 11.0v2.
The IABP (Teleflex Medical Europe Ltd., Westmeath, Ireland; Maquet Datascope Corp., Mahwah, NJ, USA; Insightra Medical Inc., Irvine, CA, USA) was implanted using the Seldinger technique via the femoral arteries under fluoroscopic or transesophageal echocardiographic guidance. Once arterial access was obtained and an introducer sheath placed, a guidewire was advanced into the descending aorta. The IABP tip was positioned 1 to 2 cm distal to left subclavian artery.
The position of the balloon was then evaluated with a chest X-ray, considering as a correct position the presence of the tip of the balloon 2 cm above the tracheal carina (8).
IABP support was used in the following conditions:
- CS, defined as systolic blood pressure <90 mmHg for >30 min or catecholamines required to maintain systolic pressure >90 mmHg plus clinical signs of pulmonary congestion (overt pulmonary edema, pulmonary rales or radiographic signs of pulmonary congestion) and impaired organ perfusion (cold and clammy skin, oliguria, altered central nervous system function) (9,10). We have included in this category, defined above “medical” CS, all causes of CS not associated with open heart surgery: CS following STEMI, acute decompensation of a chronic heart failure or new onset of heart failure, CS associated with arrhythmic storm or CS in patients undergoing cardiac interventional procedures [trans-catheter ventricular tachycardia (VT) ablation and PCI].
- IABP implantation in open-heart surgery. This category is further divided according to timing of IABP placement as follows:
- Preoperative IABP implantation in high-risk open heart surgery, including: severely impaired left ventricular function, left main coronary disease, three vessels disease, significant ongoing ischemia in a large myocardial territory, hemodynamic instability or acute myocardial infarction.
- Post-operative IABP implantation in patients who could not be weaned from cardio-pulmonary bypass or for post cardiotomy CS development, defined as the presence of: left atrial pressure (LAP) increased by >18 mmHg, cardiac index decreased to <2 L/min/m2, and mean systolic arterial pressure <90 mmHg despite adrenaline support (up to 0.2 mcg/kg/min) (9).
As per clinical protocol, IABP was invariably implanted within 1 hour from the diagnosis of CS.
During the study period, 21 patients received concomitant veno-arterial extracorporeal membrane oxygenation (V-A ECMO) and IABP implantation to provide full hemodynamic support and left ventricular unloading. All patients with ECMO were excluded from further analyses, due to the heterogeneity and peculiarity of this group of patients
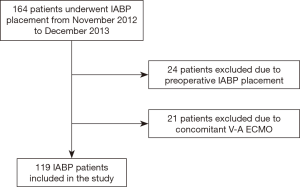
Furthermore, we have excluded from further analysis the patients who underwent prophylactic pre-operative IABP implantation, to focus our analysis only on CS patients (Figure 1).
The following variables were collected: in-hospital mortality, length of ICU stay, acute kidney injury (AKI) [defined as RIFLE (Risk of renal dysfunction, Injury to the kidney, Failure of kidney function, Loss of kidney function and End-stage kidney disease) category I (11) and cerebrovascular complications. Other clinical variables were also collected: age, sex, smoking, diabetes mellitus, hypertension, previous cardiac operations or PCI, serum creatinine level, previous myocardial infarction, peripheral vascular diseases, pulmonary arterial hypertension, cerebrovascular diseases, cardiac catheterization data, including the numbers of diseased vessels, inotropic score at the moment of IABP implantation and removal [defined as: inotropic score = dopamine dose (mcg/kg/min) + dobutamine dose (mcg/kg/min) + 100 × epinephrine dose (mcg/kg/min) + 100 × norepinephrine dose (mcg/kg/min)] (12).
IABP-related data and complications were also collected and defined as: duration of IABP support, homolateral limb ischemia, bleeding from insertion site, thoracic bleeding, embolism, vessel injury/rupture, balloon malfunction, thrombocytopenia (platelets count <50,000/mm3).
We divided ischemic limb complications as major and minor limb ischemia. Major ischemia was defined as a loss of pulse or sensation, or abnormal limb temperature or pallor, requiring surgical intervention. Minor ischemia was defined as a decrease in the arterial flow with diminished pulse resolved with balloon removal, without need of surgical intervention.
Patients on IABP were anticoagulated with heparin or bivalirudin, according to underlying disease, with an activated thromboplastin time (aPTT) target of 45–60 s, on the basis of the continuous evaluation of the thrombotic and hemorrhagic risk.
Weaning from IABP was considered when the patient achieved hemodynamic stability along with signs of organ function recovery. The weaning procedure was run through the reduction of the assistance ratio to 1:2 then 1:4 and was usually performed within 6 hours.
Transthoracic echocardiographic data were collected during the ICU stay. All examinations were performed by experienced investigators using commercially available ultrasound systems (E9; GE Vingme, Horten, Norway and iE33; Philips Medical Systems, Andover, Massachusetts). An independent investigator, not involved in IABP implantation procedure, conducted off-line echocardiographic analyses. Chamber quantifications and Doppler measurements were performed according to the criteria of the American Society of Echocardiography (13). From the apical 4- and 2-chamber views, left ventricular end-diastolic and end-systolic diameter and left ventricular ejection fraction (LVEF) were calculated by the biplane method of discs. Right ventricular (RV) function was studied through multiple parameters (tricuspid annular plane systolic excursion, tissue Doppler systolic velocity of tricuspid annulus) according to the recent recommendations of the American Society of Echocardiography (14). RV dysfunction was defined in presence of at least one of the following criteria: tricuspid annular plane systolic excursion <16 mm, tissue Doppler systolic velocity of tricuspid annulus <10 cm/s.
Statistical analysis was conducted using Stata 13 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP). Categorical variables were analyzed with chi square test and fisher exact test when appropriate. Continuous variables were compared with Student’s t-test as appropriate after visual inspection of frequency distribution. When visual inspection identified a skewed distribution, Wilcoxon rank-sum test and Kruskal Wallis test were was employed as appropriate. A probability level with P<0.05 was generally considered significant. Association of the variables with mortality was tested by Chi-square test and odds ratios (OR) with 95% confidence intervals (CI) were calculated. A logistic regression model was developed to assess the association between the effect of several risk factors on and mortality. Clinical variables associated with mortality at bivariate analysis were tested re included in the model as risk factors. A stepwise forward regression model was built retaining variables with a P<0.1 at likelihood-ratio (LR) test. A factor variable for IABP group (medical CS versus cardiac surgery) was included a priori in the model. The model was tested in terms of discrimination (using ROC curve analysis) and calibration using Hosmer-Lemeshow goodness-of-fit test. Results from the model were documented graphically in a postestimation plot.
A ROC curve analysis was conducted to identify a threshold for inotropic score at IABP implantation to discriminate for risk of mortality in this population.
Results
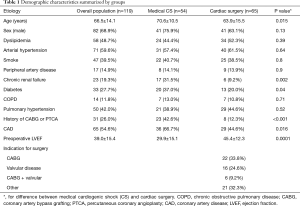
During the study period, 164 patients underwent IABP placement. After the exclusion of 24 patients who received the IABP preoperatively and those who underwent concomitant VA-ECMO and IABP support, 119 patients were included in the study (Figure 1). Demographic characteristics are reported in Table 1.
Full table
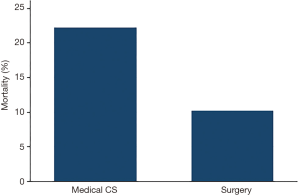
Fifty-four patients and 65 patients underwent IABP placement for medical cardiogenic or for post-cardiotomy CS, respectively. There was a borderline significant difference for mortality between medical CS [12/54 (22.2%)] and postcardiotomy patients [7/65 (10.8%)] (P=0.09, Figure 2).
Cardiac ultrasound data for each category are summarized in Table 2.

Full table
The presence of RV dysfunction was associated with an increased mortality in each group, although not statistically significant [4.8% vs. 14.6% in cardiac surgical patients (P=0.5), 18.8% vs. 30% in CS, P=0.69].
Pre-implantation LVEF was higher than post-implantation LVEF in the overall population (38.8%±14.8% vs. 31.5%±14.3%, P<0.001).
The magnitude of LV dysfunction, as expressed by LVEF, was not associated with an increased mortality in the overall population or in the subgroups of patients.
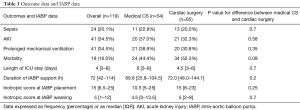
Mortality and complications for medical CS patients and for postcardiotomy patients are reported in Table 3.
Full table
The median duration of IABP support was 72 (IQR, 42–114) h, without any significant difference between groups (P=0.2), and the median ICU stay was similar between groups (P=0.7) (Table 3).
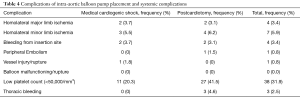
Table 4 reports the complications related to IABP treatment and the systemic complications.
Full table
The overall major morbidity rate (major limb ischemia, bleeding from insertion site, peripheral embolism, vessel injury and thoracic bleeding) was 11%. Homolateral major limb ischemia occurred in five of patients, in one case secondary to vessel injury, whereas minor limb ischemia occurred in 5.9% of patients. There were no deaths related to IABP placement.
AKI occurred in 34.5% of patients. Thrombocytopenia was more frequent in patients with longer length of IABP support [96±111 (IQR, 48–144) h in patients with thrombocytopenia vs. 48 (IQR, 24–72) h in patients without thrombocytopenia, P=0.008].
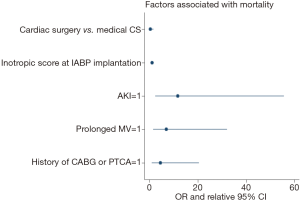
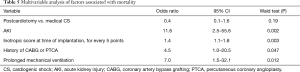
Bivariate analysis identified the following factors as associated with hospital mortality: age, peripheral vascular disease, history of CABG or PTCA, hypotension requiring inotropic support, AKI, inotropic score at the time of IABP implantation, need for blood transfusion, neurologic complications, sepsis and need for prolonged mechanical ventilation (>48 h). Multivariable analysis identified AKI (OR =11.6; 95% CI, 2.5–55.6; P=0.002), and inotropic score at the time of IABP implantation (for every 5 points OR =1.4; 95% CI, 1.1–1.8; P=0.003), and history of CABG or PTCA (OR =4.5; 95% CI, 1.0–20.5; P=0.047), and prolonged mechanical ventilation (OR =7.0; 95% CI, 1.5–32.1; P=0.012) as independent predictors for early death (P<0.05, Table 5, Figure 3). The patient group (medical CS vs. postcardiotomy patients) was not associated with mortality, after controlling for other factors (P=0.19). The model demonstrated good discrimination on ROC curve analysis [AUC =0.9 (95% CI, 0.89–0.98)], and Hosmer-Lemeshow test did not detect miscalibration (P=0.9).
Full table
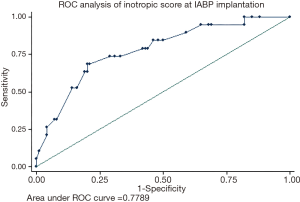
A ROC curve analysis for inotropic score at time of implantation and mortality was performed in the overall population [AUC =0.78 (95% CI, 0.66–0.90)]. A cut-off of 20 points has a specificity =71% and sensitivity =74% in this population (Figure 4).
Discussion
In our study, we have analyzed the characteristics and outcome of 119 consecutive patients who underwent IABP support for CS due to different etiologies.
In our study, mortality in patients who received IABP is not significantly different between medical CS and postcardiotomy patients. A significant difference in terms of duration of IABP support and length of ICU stay was also absent in the two groups.
In this cohort, mortality was associated with AKI development, prolonged mechanical ventilation, inotropic score at the time of IABP implantation and history of CABG.
The population we have described includes a “real world” heterogeneous series of patients, who received IABP for CS in a comprehensive MCS program. The current scenario of MCS in CS is continuously evolving and expanding, as demonstrated by a 30-fold increase in the number of percutaneous ventricular assist device implanted (15) in United States. The number of IABP implanted is still high despite its downgrading in the European and American guidelines on STEMI (1).
Our data differ under many aspects from what has been previously reported in literature. Two questions deserve careful consideration: firstly, the low mortality rate in the two group of patients; in second instance, the absence of significant differences in terms of mortality between the two groups. With regard to medical CS, the majority of studies are focused on AMI patients. Therefore, the interpretation of our results must take into account the presence of a relative low proportion of AMI that might have significantly affected the mortality in our cohort. Moreover, the inclusion of “peri-procedural” CS, as those occurred in the cath-lab, taking advantage from a timely intervention, could have contributed to the low mortality rate.
Finally, the absence of mortality differences between the two groups, being postcardiotomy shock burdened by a higher mortality rate than medical CS (10) in the majority of previous studies, might be attributed to the relatively small sample size.
The group of patients with postcardiotomy shock is worth of further comments. Postcardiotomy shock is a rare complication of cardiac surgery, associated with a high mortality rate. However, a more accurate classification is needed. Postcardiotomy shock has an incidence of 2–6% after cardiac surgery, but only 0.5–1.5% are refractory to inotropic and IABP therapy (16,17). While refractory postcardiotomy shock carries a dismal prognosis without MCS systems ensuring full cardiac function replacement, more uncertainty exists as regard to its not-refractory presentation. Therefore, due to the current lack of criteria allowing, at the time of shock onset, for an accurate discrimination of the patients’ course, caution should be used in interpreting our results. In addition, postcardiotomy shock is substantially orphan of large studies, and its management, particularly in terms of implantation of MCS, suffers from a great interinstitutional variability.
We cannot conclude if the protocol of patients’ care in our institution has contributed to the definition of better results. However, we strongly suggest a continuous evaluation, at the time of shock onset, for mechanical support candidacy. The use of IABP, in this context, should be a preliminary step. Current acquisitions on pathophysiology of CS offer a sound certainty that high doses of inotropes are detrimental on heart recovery (18). Therefore, we strongly believe that, if the end-organ perfusion is not ensured by a medium dose of inotropic support, IABP should be implanted without delay.
Current recommendations on IABP support arise from studies focused on a well-defined target population, specifically CS post AMI. The only robust RCT conducted on IABP efficacy in CS failed to found any difference in early and long term mortality in the group of IABP-supported patients. This study has raised some criticisms (19), but, in our view, pays the penalty for a homogenous cohort of patients at the expense of the realistic representation of unselected CS patients cohort. Recently, Werdan et al. summarized current indications of IABP support (10): on the basis of the most recent updates, AHA/ACC guidelines on CS after AMI recommend the use of IABP as a class IIA/A indication (4), whereas ESC guidelines state that IABP may be considered as a MCS (IIB/B) (5). The last European guidelines on myocardial revascularization further downgraded the routine use of IABP in CS associated to AMI to a class III (20).
However, having this device a strong pathophysiological basis for its benefits, and being clinicians extensively confident in this technique, the negative results might be related to other factors, such as severity of shock, timing of implantation and management.
Indeed, our study identified some prognostic parameters associated with mortality in IABP treated patients.
In the overall population, the multivariable analysis identified the development of AKI, the need for prolonged mechanical ventilation the postoperative period and the inotropic score at the moment of IABP implantation as factors associated with mortality.
The dismal effect of AKI in the prognosis of cardiac patients is nowadays a sound acquisition (21), but in this case the development of AKI is even of greater interest, as it shows that the amount of hemodynamic support is probably not sufficient and should prompt further interventions. Instead, the threshold of inotropic score we have identified sets a new paradigm for the timing of implantation. To the best of our knowledge, no previous studies suggested a threshold of pharmacological support triggering IABP implantation.
Our results might suggest that, in a certain category of patients, the IABP might be used earlier.
The interaction between LVEF variation and IABP counterpulsation is controversial. Our study suggests that the clinical improvement after IABP placement is unrelated to a LVEF increase. Even if this finding was apparently surprising, this is not a new acquisition. Indeed, a substudy of the SHOCK trial showed similar results (22) and a seminal experience demonstrated that, in patients with left ventricular failure, IABP did not improve LV performance indexes (23).
The overall IABP-related morbidity of our study (10.5%) is similar to previous reports (2,24). This feature supports the benefits of technological improvement in the devices, and reinforces the extensive use of IABP even in centers not able to provide the full panel of MCS.
Our study has the strength to represent, at the price of the heterogeneity of the population included, a “real world” picture about IABP implantation in a high-volume center. Our findings suggest a new consideration of this type of mechanical support at the time of transition from pharmacological therapies to full mechanical support.
This study has many limitations: the prospective design and the absence of control group do not allow for any inference about mortality rate nor a comparison to a baseline population without IABP. The relatively small sample size may also have contributed to the absence of any difference in terms of mortality between two greatly different groups. Moreover, the heterogeneity of the population and the relatively small number of patient included limited the power of the analysis.
Conclusions
In conclusion, even if our data do not support any inference about the efficacy of IABP, it is our opinion that the “real world” of CS is a complex scenario, not yet fully explored by the current available literature. The recent great improvement in the knowledge of pathophysiology of CS and the extraordinary technical progress in MCS should prompt further randomized studies, gathering the complexity of the disease.
Acknowledgements
We are indebted to O. G. Turla, CCP, for his continuous support and for data collection.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the Institutional Review Board of the San Raffaele Scientific Institute, Milan, Italy, and was performed in accordance with ethical principles of the Helsinki Declaration. Collection and merging of data were made in agreement with Italian and European data protection laws.
References
- Stretch R, Sauer CM, Yuh DD, et al. National trends in the utilization of short-term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol 2014;64:1407-15. [Crossref] [PubMed]
- Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287-96. [Crossref] [PubMed]
- Thiele H, Zeymer U, Neumann FJ, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet 2013;382:1638-45. [Crossref] [PubMed]
- O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:e78-140. [Crossref] [PubMed]
- Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569-619. [Crossref] [PubMed]
- Sharma S, Lumley M, Perera D. Intraaortic balloon pump use in high-risk percutaneous coronary intervention. Curr Opin Cardiol 2013;28:671-5. [Crossref] [PubMed]
- Theologou T, Bashir M, Rengarajan A, et al. Preoperative intra aortic balloon pumps in patients undergoing coronary artery bypass grafting. Cochrane database Syst Rev 2011.CD004472. [PubMed]
- Kim JT, Lee JR, Kim JK, et al. The Carina as a Useful Radiographic Landmark for Positioning the Intraaortic Balloon Pump. Anesth Analg 2007;105:735-8. [Crossref] [PubMed]
- Hausmann H, Potapov EV, Koster A, et al. Prognosis after the implantation of an intra-aortic balloon pump in cardiac surgery calculated with a new score. Circulation 2002;106:I203-6. [PubMed]
- Werdan K, Gielen S, Ebelt H, Hochman JS. Mechanical circulatory support in cardiogenic shock. Eur Heart J 2014;35:156-67. [Crossref] [PubMed]
- Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:R204-12. [Crossref] [PubMed]
- Nguyen HV, Havalad V, Aponte-Patel L, et al. Temporary biventricular pacing decreases the vasoactive-inotropic score after cardiac surgery: a substudy of a randomized clinical trial. J Thorac Cardiovasc Surg 2013;146:296-301. [Crossref] [PubMed]
- Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiograph. J Am Soc Echocardiogr 2005;18:1440-63. [Crossref] [PubMed]
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685-713. [Crossref] [PubMed]
- Kolte D, Khera S, Aronow WS, et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-Elevation myocardial infarction in the United States. J Am Heart Assoc 2014;3:e000590. [Crossref] [PubMed]
- Khorsandi M, Shaikhrezai K, Prasad S, et al. Advanced mechanical circulatory support for post-cardiotomy cardiogenic shock : a 20-year outcome analysis in a non- transplant unit. J Cardiothorac Surg 2016;11:29. [Crossref] [PubMed]
- Rastan AJ, Dege A, Mohr M, et al. Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J Thorac Cardiovasc Surg 2010;139:302-11. [Crossref] [PubMed]
- Samuels LE, Kaufman MS, Thomas MP, et al. Pharmacological Criteria for Ventricular Assist Device Insertion Following Postcardiotomy Shock: Experience with the Abiomed BVS System. J Card Surg 1999;14:288-93. [Crossref] [PubMed]
- Khashan MY, Pinsky MR. Does intra-aortic balloon support for myocardial infarction with cardiogenic shock improve outcome? Crit Care 2013;17:307. [Crossref] [PubMed]
- Kolh P, Windecker S. ESC/EACTS myocardial revascularization guidelines 2014. Eur Heart J 2014;35:3235-6. [PubMed]
- Neal JB, Shaw AD, Iv FT. Acute kidney injury following cardiac surgery : current understanding and future directions. Crit Care 2016;20:187. [Crossref] [PubMed]
- Yehudai L, Reynolds HR, Schwarz SA, et al. Serial echocardiograms in patients with cardiogenic shock: analysis of the SHOCK trial. Diagnostic Testing. J Am Coll Cardiol 2006;47:A94-157.
- Iskandrian AS, Colby J, Hakki AH, et al. Effect of intra-aortic balloon pumping on left ventricular function: evaluation by radionuclide ventriculography. Clin Cardiol 1984;7:211-6. [Crossref] [PubMed]
- Cohen M, Urban P, Christenson JT, et al. Intra-aortic balloon counterpulsation in US and non-US centres: results of the Benchmark Registry. Eur Heart J 2003;24:1763-70. [Crossref] [PubMed]