Clinical characteristics, classification and surgical treatment of periductal mastitis
Introduction
Periductal mastitis (PDM), first recognized as a “morbid condition of lactiferous ducts” by Birkett (1) in 1850, is an inflammatory condition of the subareolar ducts. Bloodgood (2) described duct dilatation and periductal inflammation as the pathological features in 1921 and recognized the disease as a distinct clinical entity 2 years later (3). In 1951, Zuska et al. described the true nature of this condition as mammary fistula and the disease was also called Zuska disease from then on (4).
The etiology of the condition remains unclear. One of the major predisposing factors is obstructed lactiferous duct (4,5). The secondary infection of the inflamed obstructed ducts often leads to duct damage and subsequent rupture with abscess formation. Recent studies indicated that smoking is another major factor in the etiology. It has been postulated to be associated with direct or indirect damage of the subareolar ducts, with tissue necrosis and subsequent infection (5-7). The breast concentrates substances in cigarette smoke; cotinine, a nicotine derivative, has higher concentrations in subareolar ducts than in plasma (8-10).
Clinically, PDM can present with nipple discharge, nipple retraction, a subareolar breast mass with or without pain, a periareolar abscess, or a mammary fistula (i.e., communication between a major subareolar duct and the skin). The subcutaneous abscess in the region of the areola usually discharges spontaneously, and then appears to resolve only to recur again (11). Empiric antibiotic therapy and wide surgical excision are the main treatments for PDM. However, the condition is still subject to recur even after sensitive antibiotic therapy and excision surgery. The recurrence rate was reported from 4.3% to 28% (12-15). Considering the different severity and extent of the disease, the recurrence rate might be influenced by different treatment modalities. Although PDM has been recognized since 1850, no accepted classification system has been proffered until now. This study aimed to describe the clinical characteristics of PDM, propose the practical clinical classification system and evaluate the results of different surgical treatments in different type of PDM patients.
Methods
Patients
In this study, we retrospectively enrolled 152 consecutive PDM patients from Peking Union Medical College Hospital between March 2012 and December 2016. Peking Union Medical College Hospital is a Class A tertiary comprehensive hospital and is designated by Chinese National Health and Family Planning Commission as one of the national referral centers offering diagnostic and therapeutic care of complex and rare disorders. Patients were included in this study if they had clinical manifestation and histopathologic confirmation of PDM including chronic recurrent PDM after at least one attempt of surgical excision. All of the enrolled patients had undergone surgery.
Breast ultrasound examination and mammography were performed on all patients before surgery not only for rule out malignancy but also for extent of the disease.
We analyzed patients’ gender, age, live birth, concomitant medical conditions, clinical presentations (mass, rubefaction, nipple retraction, abscess, skin ulceration and ductal fistula), surgical modalities and anesthesia method.
The institutional review board (IRB) of Peking Union Medical College Hospital reviewed the study and deemed it exempt from the requirement for approval. The need for informed consent was waived by IRB because of the retrospective nature of the study and the anonymous analysis of the data.
Clinical classification
According to the seriousness or involvement of the disease, we proffered an easy practical clinical four-point classification system (Table 1) in our article for the first time.
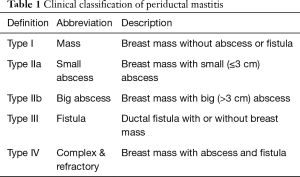
Full table
Surgical treatment
Different surgical modalities including wide surgical excision, fistulectomy and extended excision with transfer of a random breast dermo-glandular flap (BDGF) were used according to the type of PDM. All type I and II PDM received wide surgical excision. The intraoperative frozen section analyses (FSA) were performed to confirm the absence of neoplastic lesions in patients without pre-operative cytological/histological examination of the breast masses and paraffin-embedded section pathologies were also performed after surgery in all the patients. For type IIb patients, needle aspiration or incision might be needed for abscess drainage before mass excision. All type III PDM received fistulectomy, which is recognized as the Hadfield operation. Before the skin incision, a probe or tissue forcep was passed from the periareolar opening through to and out of the nipple for the precise localization of the fistula tract. The excision of fistula tract usually combined with a total duct excision (16,17). The nipple was usually cut open and nipple reconstruction was performed for such patients. For type IV PDM patients, wide excision and fistulectomy were both necessary and abscess drainage sometimes was needed before lesion wide excision. Wide excision and fistulectomy can often be performed with local anesthesia and sometimes under general anesthesia.
BDGF is a pedicled flap containing the cutaneous, subcutaneous, and mammary gland tissues, which is nourished by a number of unnamed arterioles (12). Extended excision with transfer of a random BDGF was widely used in the treatment of type IV patients with serious involvement of the whole breast especially for refractory disease. This surgery was performed under general anesthesia with laryngeal mask or endotracheal intubation. The entire lesion including infected skin, mass, abscess, fistula, inflamed ducts, and necrotic tissues were completely excised to prevent possible recurrence. The back of the nipple must be cleared of all ducts right up to the nipple skin, and a purse-string suture was often used to keep the nipple everted. The wounds of all the surgeries were thoroughly lavaged using hydrogen peroxide, iodine complex and sterile normal saline sequentially. An ipsilateral random BDGF with full-thickness breast tissue was mobilized from the underlying pectoralis major muscle and transfer to repair the breast tissue defect.
Mastectomy was used occasionally when the inflamed lesion involved nearly the whole breast or the left normal tissue after extended excision was not enough to resurface the breast tissue defect.
Follow-up
Follow-up information was obtained from outpatient clinics reviews or by telephone interviews as regularly as possible at post-operative 3, 6, 12 months and annually. Cosmetic results were divided into three categories (satisfying, acceptable and poor). The deadline of data collection was March 2017. All the authors had access to information that could identify individual patients during or after data collection.
Statistical analysis
We compared the surgery and anesthesia modalities for different types of PDM. Descriptive statistics were used for analysis because of the small numbers in some subgroups.
Results
Characteristics of patients
We studied 152 PDM patients (Table 2). Among these patients, 6 (3.9%) patients were male. The median age of this cohort was 36 years (range, 14–75 years). Twenty-three patients (23/146, 15.8%) were nulliparous. Only 4 (2.6%) patients were cigarette smokers in our study. At the time of presentation, eight patients had prolactinoma; five patients were taking antipsychotic medication and three patients had autoimmune diseases (systemic lupus erythematous, Hashimoto’s thyroiditis (HT) and autoimmune hepatitis, respectively). The mean duration of symptoms was 5.5 months (range, 0.25–84 months).
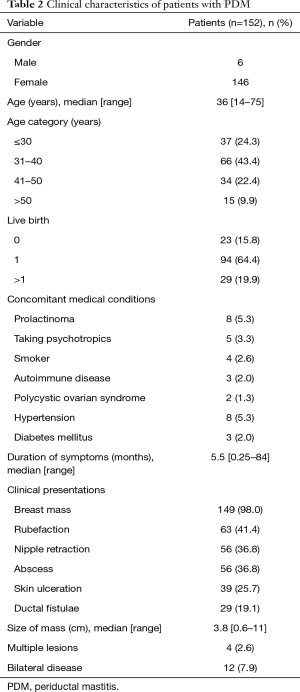
Full table
A periareolar mass was the most frequent symptom. The most common clinical manifestations were mass (98.0%), rubefaction (41.4%), nipple retraction (36.8%), abscess (36.8%), skin ulceration (25.7%), and mammary duct fistula (19.1%). The size of the mass ranged from 0.6 to 11 cm with a mean of 3.8 cm. Twelve (7.9%) of patients had bilateral involvements synchronously or asynchronously and four (2.6%) patients had multiple lesions at hospitalization. Fourteen (9.2%) patients were recurrent PDM at first hospitalization.
Classification and treatment
According to the severity and the extent of the disease, we divided the 152 patients into four types (Table 3). Almost half of the patients (78/152, 51.3%) were type I. All the type I patients underwent wide surgical excision except one patient receiving extended excision with transfer of a random BDGF because of the relatively large breast tissue defect. Nearly eighty percent of the surgeries (62/78, 79%) were performed under local anesthesia. There were eight patients highly suspected to be breast cancer before biopsy in our cohort. All of them were in type I without skin redness or swelling. Ultrasound examination showed irregular hypoechoic mass with obscure boundary in the periareolar area. The main mammography finding was an irregular focal mass in most cases.

Full table
Twenty-nine (19.1%) patients with mass and smaller abscess were classified into type IIa and 12 (7.9%) with mass and bigger abscess were in type IIb. All of the type IIa and seven of 12 type IIb patients received wide excision. Four type IIb patients underwent extended excision with BDGF transfer and one underwent mastectomy.
All of the sixteen type III patients received fistulectomy and six of them received wide mass excision at the same time.
For the seventeen type IV patients, including seven recurrent refractory PDM, the lesion was a bit more complicated. Nine wide excisions and seven extended excision with flap transfer were carried out besides fistulectomy. Fifteen surgeries were performed under general anesthesia.
Although there were 14 recurrent PDM in our study, there was only seven patients classified into type IV (complex & refractory). The remaining seven recurrent cases were evaluated to be relatively easy to deal with.
About three fifths of all the patients (93/152, 61.2%) received surgeries under local anesthesia and most of them were discharged home on the very operation day or on post-operative day (POD) 1. The patients with wound suction drainage tube were discharged home on POD 3–5.
Postoperative follow-up
A total of 145 (95.4%) patients were available for follow-up by clinic medical record and telephone interview. The median follow-up period was 36 months (range, 3–60 months). Recurrence (Table 4) in ipsilateral breast happened in 11 (7.2%) patients during follow-up and second excision surgery showed good results with one case adopting flap repair. Five of the 11 patients were type I PDM, three were type II, one was type III, and two was type IV PDM. The recurrence rates in different types were 6.4% (I), 6.9% (IIa), 8.3% (IIb), 6.3% (III) and 11.8% (IV) respectively. For the 14 initial recurrent PDMs, two cases (14%) recurred again. Among the 145 patients, 113 patients reported satisfying cosmetic results, 28 reported acceptable and 4 reported poor.

Full table
Discussion
Periductal mastitis is an uncommon inflammatory condition of the subareolar ductal system. First of all, PDM should be distinguished from duct ectasia which is characterized by distension of subareolar ducts with fibrosis (18). Originally, duct ectasia and PDM were considered part of the same clinical syndrome with PDM preceding duct ectasia. And then, duct ectasia was recognized to be a simple aberration of the normal ageing process (19) and these two diseases appeared to be separate clinical and pathological entities with different etiologies (5).
Even though PDM has been recognized for more than one century, the etiology of PDM is still poorly understood. Ductal obstruction, smoking, hyperprolactinemia, autoimmune disease, infection and trauma are possible risk factors. In most studies, the majority of PDM patients were smokers. Heavy smoking increases the risk of women developing PDM (20). But in our study, there were only 6 (4%) smokers (1 male & 5 females). The rate was just a little higher than the total smoking rate (2–3%) in Chinese females.
The relationship between PDM and prolactinoma was rarely reported. Hyperprolactinemia was demonstrated to make mammary secretion reach in lipid and protein material and simultaneously increase number of ductal epithelial cells, ductal histiocytes and macrophages, which indicated that hyperprolactinemia promoted periductal and intraductal steril inflammation (21). There were eight patients (5.3%) in our study who had prolactinoma at first presentation. Besides prolactinoma, many antipsychotic agents are known to increase prolactin (PRL) secretion because of their inhibitory effect of dopamine. Psychotropics-induced hyperprolactinemia was reported to be associated with idiopathic granulomatous mastitis, which is another kind of non-lactational mastitis, occasionally (22,23). To the best of our knowledge, psychotropics associated PDM has not been reported yet. In our study, there were five patients taking antipsychotic medications. Considering not all patients with hyperprolactinemia developed PDM. The high PRL (HPRL) level may be a trigger for PDM. PRL is not only secreted from the pituitary gland but also from other organs and cells particularly lymphocytes. PRL was reported to have an immune stimulatory effect and promote autoimmunity. In the last two decades many multi-organ and organ specific autoimmune diseases including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), Sjogren’s syndrome (SS), HT and multiple sclerosis (MS) were discussed to be associated with HPRL (24). In our study, there were three patients having autoimmune disease (SLE, HT and autoimmune hepatitis, respectively). But unfortunately, the serum PRL level was not tested. It is not clear whether the autoimmune disease and PDM are both the manifestations of the disordered immune system or autoimmune disease brings about PDM. Elevated PRL serum levels were also documented in the early studies of patients with polycystic ovaries syndrome. However, currently, there is no evidence of a pathophysiological link between these two entities (25,26). Although there are only two PDM patients having polycystic ovaries syndrome in our patient series, the relationship in these two entities still need further investigation.
Periductal mastitis primarily affects young women but can occur in men as well. Several studies have reported PDM in males (27-29). And the six male cases in our study could be a supplementary belonging to this group.
The main manifestations of PDM include periareolar mass, abscess and ductal fistula. Some of the masses are painful with skin red and swollen. Some of them are just painless lumps without any discomfort. Nearly all of our patients had masses, and more than one-third patients had abscesses or fistulae. The different manifestations are the real clinical presentations of PDM in differential stage. A breast mass with mild underlying inflammatory changes might be the first stage. And then single or multiple inflammatory masses show up. If inflammation is not controlled, abscess formation is coming. The initial management of subareolar abscess consists of antibiotic therapy and abscess drainage. But if the underlying periductal mastitis is not removed, abscess recurs in the same location and discharges at the edge of the areola spontaneously. Thus, a periductal fistula is formed eventually. It is necessary for us to take the seriousness and involvement of the disease into account when we are discussing about the disease treatment. So, we proffered the PDM clinical classification system for the first time as far as we know.
For different situation, we choose different treatment modality. The proper treatment modality is the guarantee of low recurrence rate. For type I PDM (mass without abscess or fistula), non-surgical treatment such as the use of antibiotics is usually the first choice if pre-operative cytological/histological diagnosis is available. But, unfortunately, it is not realistic to do core needle biopsy (CNB) for every patient in China especially in rural area in the economic view of point, because the cost of CNB cannot be totally covered by basic medical insurance. With the considering of the limited medical expenditure of excision biopsy in China, mass excision is not a bad choice for patients to get diagnosis and removal of the lesion at the same time especially when the lesion simulated a breast cancer. So, for type I and type II PDM, wide surgical excision is often used. For type IIb patients, abscess drainage is recommended before mass excision and flap repair is needed when the tissue defect is too large to suture up directly. For type III PDM, fistulectomy (11,30-33) is deemed to be effective with favorable cosmetic result. For type IV PDM, fistulectomy and wide excision are both necessary. No matter non-complicating PDM or recurrent refractory PDM, the complete debridement is the key for surgical treatment. The resection margin is required to be at least 0.5 cm from the inflamed tissues. In our study, the recurrence rates of type I to III were about 7%. The mean size of recurrent mass was 5.2, 4.0 and 11 cm for type I, IIa, and IIb respectively, which was obviously larger than the mean size of the whole study cohort except one type III case. Among the 17 type IV patients, two got recurrence. All these recurrent cases received wide excision without flap repair and most of them were under general anesthesia. The most possible reason for recurrence is still the insufficient resection margin. With the consideration of the unclear etiology of PDM, the left inflamed tissue and associated cytokines maybe a trigger for the recurrence. We cannot emphasize too much the importance of the complete debridement of inflamed tissue especially for initial recurrent PDM. A full grasp of the extent of inflammation is the prerequisite for complete resection. Breast ultrasound and mammography were performed routinely before surgery not only for rule out malignancy but also for extent of the disease. Ultrasound can distinguish inflammatory mass and purulent cavity more accurately than mammography. Magnetic resonance imaging scan were occasionally performed especially in type IV patients to provide more detail information about the inflammation extent.
For large breast tissue defect patients, extended excision with BDGF is strongly recommended. None of the patients in our study receiving BDGF repair recurred. In Zhang et al.’s study (12), the recurrence rate of this surgical modality was only 4.3%. Low et al. (34) ever reported a technique, involving use of the pectoralis major muscle flap, for treating refractory PDM in three patients. However, the surgical technique for muscle flap harvest and transfer is more complicated than breast tissue flap and the risk for surgical morbidities is also higher with muscle flap.
From the results of our study, the total recurrence rate can be controlled to 7.2% with the proper surgical modality according to different type, which is much lower than the rate ever reported (13-15). The classification system also provides some information for PDM outcomes, with type IV PDM having a bitter higher recurrence rate (11.8%) than other non-complicating types (6.3–8.3%).
Our study has several limitations. First, due to the retrospective nature of the study, the clinical data for PDM patients were a bit limited especially for outpatients, so we didn’t choose non-surgical PDM outpatients in the same period as control group. And many patients in our hospital are from rural area. The medical condition for Chinese rural area is relatively poor. The repeated medical treatment after PDM relapse will seriously affect the daily life and productivity of rural populations. These patients seek for a shorter treatment cycle, and so, surgical treatment with low recurrence rate becomes a favorable choice in our study. Second, as far as we know, the classification of the PDM was proposed for the first time according to the severity and the extent of the disease, the rationality and practicability of the classification system is still worth discussing. Third, the follow-up period was not long enough for better recurrent rate analysis. We will follow up all patients in this study in the following years to get more recurrent information.
Conclusions
Periductal mastitis is a distinct breast inflammatory condition of unknown etiology. Mass, abscess and fistula are most common manifestations of the disease. The four-point clinical classification was first proffered according to different manifestations. Different surgical modalities including wide surgical excision, fistulectomy and extended excision with transfer of a random BDGF are effective surgical modalities with low recurrence rates and good cosmetic results for different type of PDM.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The institutional review board (IRB) of Peking Union Medical College Hospital reviewed the study and deemed it exempt from the requirement for approval. The need for informed consent was waived by IRB because of the retrospective nature of the study and the anonymous analysis of the data.
References
- Birkett J. The diseases of the breast and their treatment. London: Longmans, 1850.
- Bloodgood JC. Pathology of chronic cystic mastitis of female breasts: With special consideration of blue-domed cysts. Arch Surg 1921;3:445-52. [Crossref]
- Bloodgood JC. The clinical picture of dilated ducts beneath the nipple frequently to be palpated as a doughy wormlike mass-The varicocele tumour of the breast. Surg Gynecol Obstet 1923;36:486-95.
- Zuska JJ, Crile G Jr, Ayres WW. Fistulas of lactifierous ducts. Am J Surg 1951;81:312-7. [Crossref] [PubMed]
- Dixon JM, Ravisekar O, Chetty U, et al. Periductal mastitis and duct ectasia: different conditions with different aetiologies. Br J Surg 1996;83:820-2. [Crossref] [PubMed]
- Dixon JM. Periductal mastitis: aetiology and management. Br J Clin Pract Suppl 1989;68:76-80. [PubMed]
- Bundred NJ. The aetiology of periductal mastitis. Breast 1993;2:12. [Crossref]
- Schäfer P, Fürrer C, Mermillod B. An Association of Cigarette Smoking with Recurrent Subareolar Breast Abscess. Int J Epidemiol 1988;17:810-3. [Crossref] [PubMed]
- Bundred NJ, Dover MS, Coley S, et al. Breast abscesses and cigarette smoking. Br J Surg 1992;79:58-9. [Crossref] [PubMed]
- Hill P, Wynder EL. Nicotine and cotinine in breast fluid. Cancer Lett 1979;6:251-4. [Crossref] [PubMed]
- Taffurelli M, Pellegrini A, Santini D, et al. Recurrent periductal mastitis: Surgical treatment. Surgery 2016;160:1689-92. [Crossref] [PubMed]
- Zhang X, Lin Y, Sun Q, et al. Dermo-glandular flap for treatment of recurrent periductal mastitis. J Surg Res 2015;193:738-44. [Crossref] [PubMed]
- Li S, Grant CS, Degnim A, et al. Surgical management of recurrent subareolar breast abscesses:Mayo Clinic experience. Am J Surg 2006;192:528-9. [Crossref] [PubMed]
- Versluijs-Ossewaarde FN, Roumen RM, Goris RJ. Subareolar breast abscesses: characteristics and results of surgical treatment. Breast J 2005;11:179-82. [Crossref] [PubMed]
- Dixon JM. Mammary duct ectasia-periductal mastitis complex. Br J Surg 1996;83:1017-9. [Crossref] [PubMed]
- Komenaka IK, Pennington RE Jr, Bowling MW, et al. A Technique to Prevent Recurrence of Lactiferous Duct Fistula. J Am Coll Surg 2006;203:253-6. [Crossref] [PubMed]
- Almasad JK. Mammary duct fistulae:classification and management. Anz J Surg 2006;76:149-52. [Crossref] [PubMed]
- Dixon JM. Periductal mastitis/duct ectasia. World J Surg 1989;13:715-20. [Crossref] [PubMed]
- Hughes LE, Mansel RE, Webster DJ. Aberrations of normal development and involution (ANDI): a new perspective on pathogenesis and nomenclature of benign breast disorders. Lancet 1987;2:1316-9. [Crossref] [PubMed]
- Bundred NJ, Dover MS, Aluwihare N, et al. Smoking and periductal mastitis. BMJ 1993;307:772-3. [Crossref] [PubMed]
- Radojković D, Antić S, Pesić M, et al. Significance of hyperprolactinemia for cytomorphologic features of breast secretions. Vojnosanit Pregl 2010;67:42-7. [Crossref] [PubMed]
- Kutsuna S, Mezaki K, Nagamatsu M, et al. Two Cases of Granulomatous Mastitis Caused by Corynebacterium kroppenstedtii Infection in Nulliparous Young Women with Hyperprolactinemia. Intern Med 2015;54:1815-8. [Crossref] [PubMed]
- Lin CH, Hsu CW, Tsao TY, et al. Idiopathic granulomatous mastitis associated with risperidone-induced hyperprolactinemia. Diagn Pathol 2012;7:2. [Crossref] [PubMed]
- Shelly S, Boaz M, Orbach H. Prolactin and autoimmunity. Autoimmun Rev 2012;11:A465-70. [Crossref] [PubMed]
- Robin G, Catteau-Jonard S, Young J, et al. Physiopathological link between polycystic ovary syndrome and hyperprolactinemia: myth or reality? Gynecol Obstet Fertil 2011;39:141-5. [Crossref] [PubMed]
- Bracero N, Zacur HA. Polycystic ovary syndrome and hyperprolactinemia. Obstet Gynecol Clin North Am 2001;28:77-84. [Crossref] [PubMed]
- Al-Masad JK. Mammary duct ectasia and periductal mastitis in males. Saudi Med J 2001;22:1030-3. [PubMed]
- Kim BS, Lee JH, Kim WJ, et al. Periductal mastitis mimicking breast cancer in a male breast. Clin Imaging 2013;37:574-6. [Crossref] [PubMed]
- Palmieri A, D’Orazi V, Martino G, et al. Plasma Cell Mastitis in Men:A Single-center Experience and Review of the Literature. In Vivo 2016;30:727-32. [Crossref] [PubMed]
- Hadfield J. Excision of the major duct system for benign disease of the breast. Br J Surg 1960;47:472-7. [Crossref] [PubMed]
- Bundred NJ, Dixon JM, Chetty U, et al. Mammillary fistula. Br J Surg 1987;74:466-8. [Crossref] [PubMed]
- Meguid MM, Oler A, Numann PJ, et al. Pathogenesis-based treatment of recurring subareolar breast abscesses. Surgery 1995;118:775-82. [Crossref] [PubMed]
- Móricová P, Žúbor P, Kapustová I, et al. Recurrent subareolar non puerperal abscess of breast with fistules of lactiferous ducts (Zuskas disease). Rozhl Chir 2013;92:509-11. [PubMed]
- Low N, Barry PA. Pectoralis major interposition flap: A new technique for treatment of severe peri-ductal mastitis. Breast 2009;18:115-8. [Crossref] [PubMed]


