A lower level of forced expiratory volume in one second predicts the poor prognosis of small cell lung cancer
Introduction
Small cell lung cancer (SCLC) accounts for 10–15% of all lung cancers, although the incidence of SCLC has been declining with the decreasing prevalence of smoking (1). SCLC is distinct from non-small cell lung cancer (NSCLC) by its aggressive and rapid-growing features (2). Despite its dramatic initial response to chemotherapy and radiation, SCLC remains associated with poor long-term clinical outcomes. Many putative prognostic factors have been suggested, and the most important factors are poor performance status, weight loss and staging of extensive disease (ED) (3). According to Paesmans et al., age and gender are also independent prognostic factors in SCLC (4).
Smoking is a major risk factor for SCLC (5). The proportion of smokers among SCLC patients is approximately 95%. Chronic obstructive pulmonary disease (COPD) mediates the higher effects of smoking behaviors on SCLC risk. Compared with non-COPD patients, the risk of SCLC was 1.86 times higher in COPD patients (6). Smoking exposure and the loss of forced expiratory volume in 1 second (FEV1) are associated with the development of lung cancer (7,8). The airway obstruction and treatment response of lung cancer is controversial. In early-stage lung cancer, emphysema, not airway obstruction, is associated with poor prognostic outcomes (9). But, the combination of COPD and cancer stage, age, gender and tumor grade improves the prediction of treatment response in NSCLC (10). Reduced FEV1 is strongly associated with mortality in patients with advanced NSCLC (11). However, few studies have evaluated the impairment of pulmonary function and the presence of COPD as a prognostic factor in SCLC, though smoking is a very strong risk factor for the development of SCLC, which shares common mechanistic pathway with smoking behaviors and airway obstruction (6).
In our study, we aimed to investigate whether the parameters of impaired lung function are associated with clinical outcomes in patients with SCLC.
Methods
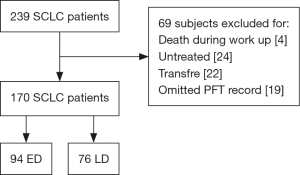
This was a multicenter retrospective study conducted in Bucheon St. Mary’s Hospital, St. Paul’s Hospital, Uijeongbu St. Mary’s Hospital, and Incheon St. Mary’s Hospital in the Republic of Korea from 2011 to 2015. All consecutive patients that were diagnosed with SCLC and treated with chemotherapy and/or radiotherapy were included. Patients who lacked pulmonary function test (PFT) data, died during staging work up, were untreated, or who transferred out to other centers were excluded. The study flow is summarized in Figure 1. A PFT was performed on all patients at the time of lung cancer work up for diagnosis and staging and a COPD diagnosis was confirmed by an incompletely reversible airflow limitation [post-bronchodilator FEV1 to forced vital capacity (FVC) ratio of <0.7] (12). PFT maneuver was performed to meet the repeatability criteria (13). In our study, 93.0% of all patients met repeatability criteria. If the criteria are not met, the test was repeated up to 8 times. If the patient was unable to continue the examination, or was asked not to do any further tests, the three best tests were selected when it was judged that the results were difficult to obtain even if the tests were added. All data were collected from hospital databases. The requirement for informed consent was waived by the institutional review boards because the study was based on retrospective chart reviews. This study was approved by the institutional review boards of all participating centers (IRB No. XC17REDI0038).
Diagnostic procedure and treatment
All patients were evaluated for their performance status to define the extent of their disease which included a bronchoscopy, computed tomography (CT) scan of the chest, bone scan, positron emission tomography CT, and brain magnetic resonance imaging. Patients were classified into either a limited disease (LD) or an ED group (14). Treatment included chemotherapy, radiotherapy, and concurrent chemo-radiotherapy (CCRT). First-line chemotherapy included platinum-based chemotherapy with etoposide or irinotecan or belotecan. The overall survival (OS) was defined as the period of time from diagnosis to the time of death.
Data
We extracted the following data from patients’ medical records: patient demographics; smoking history; stage of lung cancer; Eastern Cooperative Oncology Group (ECOG) performance status; history of chemotherapy and/or radiation; PFT results; survival status; and, the date of death for patients with a follow-up loss (15).
Statistical analysis
We used Pearson’s chi-squared tests to compare discrete variables and Student’s t-tests to compare continuous variables. A Kaplan-Meier univariate analysis for categorical variables and a univariate Cox proportional hazards regression for continuous variables were used to analyze the impact of possible predictors on survival rates. Hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs) were calculated for the predictors that were significant in the multivariate analysis. A two-sided P value of less than 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS for Windows software (ver. 20.0; IBM Corp., Armonk, NY, USA).
Results
Overall, 239 patients were diagnosed with SCLC with 69 meeting the exclusion criteria of death during staging work-up, no chemotherapy and/or radiotherapy, transferal to another medical center, or lack of a PFT record. Thus, 170 patients (94 ED and 76 LD) were ultimately included in our analysis. Of these patients, 93 and 77 patients were classified into the COPD and non-COPD groups. We compared the two groups and explored the clinical factors that predicted OS based on the lung function impairment measured at baseline.
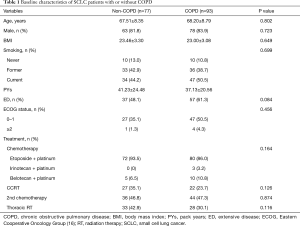
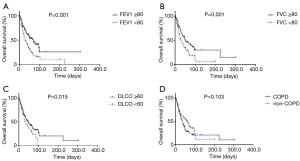
The clinical characteristics of COPD and non-COPD groups were compared. Baseline characteristics, including age, gender, body mass index, history of smoking, staging, ECOG status, and treatment strategies, did not differ significantly between the two groups (Table 1). Patients with a FEV1 less than 80% predicted or FVC less than 80% predicted displayed a shorter median OS than those with a FEV1 of 80% or more predicted or FVC of 80% or more predicted (280.6±60.44 vs. 697.0±133.57 days, P=0.001; 244.0±38.27 vs. 491.0±104.01 days, P=0.001), respectively (Figure 2A,B). In addition, patients with a diffusing capacity of the lungs for carbon monoxide (DLCO) less than 60% demonstrated a shorter median OS than those with a DLCO of 60% or more (295.0±59.77 vs. 480.0±150.72 days, P=0.015) (Figure 2C). However, the median OS was not different between the COPD and non-COPD groups (365.0±47.45 vs. 618±126.12 days, P=0.103) (Figure 2D).
Full table
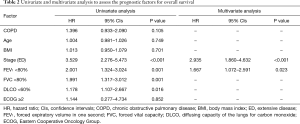
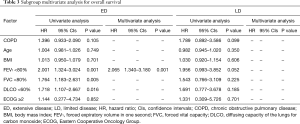
A multivariate analysis revealed that ED (HR =2.935, P<0.001) and a FEV1 less than 80% (HR =1.667, P=0.023) were independent negative prognostic factors of OS (Table 2). In the subgroup multivariate analysis, a FEV1 less than 80% (HR =2.065, P=0.001) was independently associated with poor OS in SCLC patients with ED (Table 3).
Full table
Full table
Discussion
This study investigated the clinical outcomes of SCLC patients according to the impairment of lung function as measured at baseline. A lower FEV1 and ED were significant poor prognostic factors for OS. However, COPD was not associated with mortality. In the subgroup analysis, a lower FEV1 was the only independent poor prognostic factor for OS in ED patients.
In our study, COPD status and OS was not significantly related in SCLC patients. It is known that COPD had significantly worse clinical outcomes than those without COPD in NSCLC patients. The prognostic effect of COPD in squamous cell lung carcinoma was relatively stronger than that in adenocarcinoma. The difference in the prognostic effects according to the cell types in NSCLC may be explained by a history of smoking and because COPD is closely related to squamous cell lung carcinoma than adenocarcinoma (10). Although smoking has a larger effect on SCLC risk among COPD patients (6), to our knowledge, the impact of COPD status on the prognosis in SCLC patients has not been previously examined.
Regarding the lung function parameters, a lower FEV1 was associated with shorter OS in our study. A reduced FEV1 is not only the marker of severity of COPD but also a predictor of increased risk of lung cancer, cardiovascular disease and premature death from all causes in the general population (17-19). In patients with lung cancer, a reduced FEV1 was strongly associated with mortality in advanced NSCLC and a low predicted postoperative FEV1 was related to mortality in completely resected NSCLC (11,20).
An interesting finding which deserves further exploration is that a lower FEV1, not COPD, was associated with poor treatment in SCLC patients. This finding can be explained by the patients with a ‘restrictive pattern’ in spirometry (53.2%, 41/77) that were included in the non-COPD group (data not shown). In our study, restrictive lung disease could not be confirmed because all patients were not performed additional lung volume measurement. The current definition of COPD depends on the presence of the spirometry measures of airflow obstruction. However, not all current and ex-smokers without airflow obstruction in spirometry are free of disease. Patients with a smoking history without airflow obstruction may have severe respiratory symptoms and are at risk for poor clinical outcomes (21).
Reduced FVC, a lung function parameter that suspect restrictive lung disease, was negatively associated with OS in an univariate analysis even though FVC was not found to be an independent prognostic predictor of long-term survival. A reduced FVC has been known to be one of the risk factors for total and cardiovascular disease mortality (22). A recently published study demonstrated that a reduced FVC is associated with arterial stiffness, one of the cardiovascular risk factors, in both genders (23). In lung cancer, FVC is an independent prognostic predictor for long-term survival after curative resection (24). To investigate the relationship between restrictive lung disease and poor clinical outcomes of SCLC, further study including lung volume measurement is needed.
A low DLCO is known to be associated with CT-detected emphysema and a high risk of dying from lung cancer in COPD patients (25). Chemotherapy and radiotherapy induced pulmonary toxicity that deteriorate a DLCO has been reported in NSCLC (26,27). The addition of chemotherapy to RT significantly exacerbates the decrease of DLCO in NSCLC (28). However, in our study, the Cox regression analysis failed to show that a low DLCO is an independent factor for mortality. Yet, HR was higher for the subjects with a DLCO less than 60% in a univariate analysis.
This study had several limitations. First, the work was retrospective in nature. Nonetheless, this was a multi-center study with a large sample size, which allowed us to have greater power to detect the relationship between impairment of lung function and prognosis in SCLC patients, and the risk of poorer clinical outcomes in more homogeneous subgroups. Second, the scope of this study was limited to PFT and did not address the role of emphysema or an endobronchial lesion in determining the poor clinical outcomes of SCLC (25,29). Third, additional treatments for COPD such as inhalers or anti-inflammatory agents were not considered. A prospective study is needed to clarify the clinical prognostic factors for SCLC.
Despite these limitations, to the best of our knowledge, the examination of the impact of airway obstruction on clinical outcomes in SCLC patients has not been previously performed. Spirometry is a simple and reliable tool to evaluate lung functions in clinical practice and this information could help clinicians predict treatment outcomes in SCLC patients.
In conclusion, low FEV1, not COPD, was associated with mortality in SCLC patients treated with chemotherapy and/or radiotherapy. A pretreatment spirometry in SCLC may assist in predicting the prognosis of SCLC. Further study is needed to clarify whether additional treatments for airway obstruction such as inhalers affect the clinical outcomes in SCLC patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional review boards of all participating centers (IRB No. XC17REDI0038).
References
- Kalemkerian GP, Schneider BJ. Advances in Small Cell Lung Cancer. Hematol Oncol Clin North Am 2017;31:143-56. [Crossref] [PubMed]
- Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539-44. [Crossref] [PubMed]
- Stupp R, Monnerat C, Turrisi AT 3rd, et al. Small cell lung cancer: state of the art and future perspectives. Lung Cancer 2004;45:105-17. [Crossref] [PubMed]
- Paesmans M, Sculier JP, Lecomte J, et al. Prognostic factors for patients with small cell lung carcinoma: analysis of a series of 763 patients included in 4 consecutive prospective trials with a minimum follow-up of 5 years. Cancer 2000;89:523-33. [Crossref] [PubMed]
- Freedman ND, Leitzmann MF, Hollenbeck AR, et al. Cigarette smoking and subsequent risk of lung cancer in men and women: analysis of a prospective cohort study. Lancet Oncol 2008;9:649-56. [Crossref] [PubMed]
- Huang R, Wei Y, Hung RJ, et al. Associated Links Among Smoking, Chronic Obstructive Pulmonary Disease, and Small Cell Lung Cancer: A Pooled Analysis in the International Lung Cancer Consortium. EBioMedicine 2015;2:1677-85. [Crossref]
- Wieshammer S, Dreyhaupt J. Smoking exposure, loss of forced expiratory volume in one second and the risk of lung cancer among patients with malignant disease who present with cardiac or pulmonary symptoms: a cross-sectional study. Tob Induc Dis 2017;15:16. [Crossref] [PubMed]
- Tockman MS, Anthonisen NR, Wright EC, et al. Airways obstruction and the risk for lung cancer. Ann Intern Med 1987;106:512-8. [Crossref] [PubMed]
- Ueda K, Jinbo M, Li TS, et al. Computed tomography-diagnosed emphysema, not airway obstruction, is associated with the prognostic outcome of early-stage lung cancer. Clin Cancer Res 2006;12:6730-6. [Crossref] [PubMed]
- Putila J, Guo NL. Combining COPD with clinical, pathological and demographic information refines prognosis and treatment response prediction of non-small cell lung cancer. PLoS One 2014;9:e100994. [Crossref] [PubMed]
- Lee JH, Song EM, Sim YS, et al. Forced expiratory volume in one second as a prognostic factor in advanced non-small cell lung cancer. J Thorac Oncol 2011;6:305-9. [Crossref] [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Diseases (2017 Report): Global Initiative for Chronic Obstructive Lung Disease, Inc. 2017. Available online: http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [Crossref] [PubMed]
- Kalemkerian GP, Akerley W, Bogner P, et al. Small cell lung cancer. J Natl Compr Canc Netw 2013;11:78-98. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. [Crossref] [PubMed]
- Menezes AM, Pérez-Padilla R, Wehrmeister FC, et al. FEV1 is a better predictor of mortality than FVC: the PLATINO cohort study. PLoS One 2014;9:e109732. [Crossref] [PubMed]
- Young RP, Hopkins R, Eaton TE. Forced expiratory volume in one second: not just a lung function test but a marker of premature death from all causes. Eur Respir J 2007;30:616-22. [Crossref] [PubMed]
- Shibata Y, Inoue S, Igarashi A, et al. A lower level of forced expiratory volume in 1 second is a risk factor for all-cause and cardiovascular mortality in a Japanese population: the Takahata study. PLoS One 2013;8:e83725. [Crossref] [PubMed]
- Varela G, Novoa N, Jiménez MF. Influence of age and predicted forced expiratory volume in 1 s on prognosis following complete resection for non-small cell lung carcinoma. Eur J Cardiothorac Surg 2000;18:2-6. [Crossref] [PubMed]
- Martinez CH, Murray S, Barr RG, et al. Respiratory Symptoms Items from the COPD Assessment Test Identify Ever-Smokers with Preserved Lung Function at Higher Risk for Poor Respiratory Outcomes. An Analysis of the Subpopulations and Intermediate Outcome Measures in COPD Study Cohort. Ann Am Thorac Soc 2017;14:636-42. [Crossref] [PubMed]
- Gray L, Hart CL, Smith GD, et al. What is the predictive value of established risk factors for total and cardiovascular disease mortality when measured before middle age? Pooled analyses of two prospective cohort studies from Scotland. Eur J Cardiovasc Prev Rehabil 2010;17:106-12. [Crossref] [PubMed]
- Wu IH, Sun ZJ, Lu FH, et al. Restrictive Spirometry Pattern is Associated with Increased Arterial Stiffness in Men and Women. Chest 2017;152:394-401. [Crossref] [PubMed]
- Guo X, Cao H, Xu J, et al. Forced vital capacity predicts long-term survival for curative-resected NSCLC. Med Oncol 2014;31:146. [Crossref] [PubMed]
- de-Torres JP, Marín JM, Casanova C, et al. Identification of COPD Patients at High Risk for Lung Cancer Mortality Using the COPD-LUCSS-DLCO. Chest 2016;149:936-42. [Crossref] [PubMed]
- Maas KW, van der Lee I, Bolt K, et al. Lung function changes and pulmonary complications in patients with stage III non-small cell lung cancer treated with gemcitabine/cisplatin as part of combined modality treatment. Lung Cancer 2003;41:345-51. [Crossref] [PubMed]
- Miller KL, Zhou SM, Barrier RC Jr, et al. Long-term changes in pulmonary function tests after definitive radiotherapy for lung cancer. Int J Radiat Oncol Biol Phys 2003;56:611-5. [Crossref] [PubMed]
- Gopal R, Starkschall G, Tucker SL, et al. Effects of radiotherapy and chemotherapy on lung function in patients with non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2003;56:114-20. [Crossref] [PubMed]
- Chou PC, Lin SM, Lo CY, et al. Endobronchial mucosa invasion predicts survival in patients with small cell lung cancer. PLoS One 2012;7:e47613. [Crossref] [PubMed]


