Present indications of surgical exploration of the mediastinum
Introduction
The current American and European guidelines for preoperative mediastinal nodal staging for non-small cell lung cancer (NSCLC) (1,2) are in agreement in obtaining the highest certainty level before lung resection. For this reason, their recommendation is to get tissue confirmation of regional nodal spread in all cases except in those patients with small (≤3 cm) peripheral carcinomas with no evidence of nodal involvement on computed tomography (CT) and positron emission tomography (PET). Tissue confirmation can be performed with endoscopic or surgical techniques. When both modalities are available, it is recommended to start with endobronchial ultrasonographic fine-needle aspiration (EBUS-FNA) or with esophageal ultrasonographic FNA (EUS-FNA) or with their combination. Nevertheless, their negative results should be validated with a mediastinoscopy, technique that still remains the gold standard for preresection mediastinal assessment. Other surgical procedures [such as extended cervical mediastinoscopy (ECM), parasternal mediastinotomy or thoracoscopy] can be chosen depending on the location of suspicious nodes, the surgeon’s preference or the policy of each surgical team.
The aim of this review is to describe the present indications of the classical and most innovative surgical techniques for staging NSCLC, and their integration in the current staging algorithms according on their accuracy in the different clinical scenarios.
Mediastinoscopy
Carlens described his first report on mediastinoscopy in 1959 after performing more than 100 procedures without any complications (3). For more than a half a century, this procedure was considered the gold standard for mediastinal nodal assessment. At the present time, despite the introduction of other less invasive procedures that provide cyto-histological evidence of nodal status, mediastinoscopy still has an important role in invasive staging of mediastinal lymph nodes, not only in patients with lung cancer, but also in those with mesothelioma and with potentially resectable lung metastases with radiological or metabolic suspicion of mediastinal nodal disease.
Mediastinal exploration range
During mediastinoscopy, the mediastinoscope follows the whole length of the trachea and bronchi allowing the exploration of the superior and middle mediastinum. For lung cancer staging, the range of exploration includes the cervical lymph nodes of the sternal notch, the lymph nodes along the trachea and both main bronchi, that is, the superior and inferior, left and right, paratracheal lymph nodes (nodal stations 2R, 2L, 4R, 4L, respectively), the subcarinal nodes (4) and the right and left hilar lymph nodes (nodal stations 10R, 10L, respectively) (Table 1), according to the International Association for the Study of Lung Cancer (IASLC) lymph node map (9).

Full table
Mediastinoscopy in the current staging algorithms for NSCLC
Role of mediastinoscopy for staging locally advanced NSCLC
There is a risk of mediastinal nodal involvement of at least 60% when tumours are classified as clinical (c) N2–3 on PET-CT (10). For this reason, it is recommended to confirm pathologically any mediastinal abnormality detected on the chest CT and or PET. Based on the results of several recent randomized controlled trials (4,11,12), the best strategy for mediastinal nodal staging is to start with an endosonography method (EBUS-FNA, EUS-FNA or their combination). If these explorations are positive for cancer, the information may be adequate to start a multidisciplinary treatment protocol. However, endosonography procedures have a high post-test probability (>0.10) and, therefore, their negative results should be validated with a confirmatory mediastinoscopy (4,12,13) (Table 2).
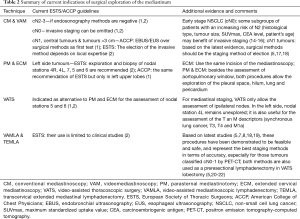
Full table
Role of mediastinoscopy for staging early stage NSCLC
According on the current European Society of Thoracic Surgeons (ESTS) guidelines, preoperative invasive mediastinal staging can be omitted if all the following criteria apply: (I) primary tumour located in the outer third of the lung; (II) largest diameter of the tumour is ≤3 cm; (III) absence of intrathoracic lymph node(s) on CT and PET (2). The rationale is that, in this situation, the rate of unsuspected pathologic mediastinal nodal disease is <10% (2,5,23,24). Nevertheless, it is important to have in mind that the current clinical TNM classification is only based on anatomical characteristics of the tumour defined by imaging and metabolic techniques. The heterogeneity of tumours included in the early stage is high because several histological types and different patient’s clinical parameters are at play. Some authors have reported that, in early stage NSCLC, the combination of tumour characteristics [histological type, consolidation/tumour ratio, tumour size, maximum standardized uptake value (SUVmax value)] and other clinical parameters [serum carcinoembriogenic antigen (CEA) level, patient’s age] have an increased risk of mediastinal involvement (33.8%) (14,15). Therefore, despite the fact that current indications of invasive mediastinal staging are limited, some subgroups of patients with an increased risk may benefit from it (16) (Table 2).
Regarding the accuracy of invasive mediastinal staging methods in this type of patients (clinical N0 disease by PET-CT), minimally invasive endoscopic techniques have a poor sensitivity (0.17–0.41) (25-28) and the sensitivity and negative predictive value (NPV) of mediastinoscopy is investigator dependent, and accounts for the reported heterogeneity of 0.32 to 0.97 and 0.8 to 0.99, respectively (1,29) (Table 1). Transcervical lymphadenectomies [video-assisted mediastinoscopic lymphadenectomy (VAMLA) or transcervical extended mediastinal lymphadenectomy (TEMLA)] are the only pre-surgical staging procedures with the highest sensitivity and NPV reported to date for those patients without suspicion of N2 by PET-CT: 0.88–0.96 and 0.94–0.99, respectively (5-7,30) (Tables 1,2). Taking into account the low prevalence of unsuspected mediastinal nodal disease in this scenario and that up to 80% of this patients with unsuspected N2 disease have single station N2 disease (31), some authors suggest that VAMLA or TEMLA should be most valuable at the time of resection instead of being a pre-surgical staging technique (32).
Role of mediastinoscopy for staging tumours with intermediate suspicion of N2–3 disease
Tumours considered with an intermediate suspicion of N2–3 disease and normal mediastinum by CT and PET are the following: central tumour or cN1 tumours (1). The rate of unsuspected pathologic mediastinal nodal disease for this subgroup ranges from 20% to 42% (5,17,18,33-35). Hence, current preoperative staging guidelines recommend invasive staging of the mediastinum over the imaging alone (1,2). However, there is a little disagreement between American and European guidelines about the best staging procedure to start with. The American College of Chest Physicians (ACCP) guidelines suggest endosonography methods over surgical procedures as the best first test (level of evidence 2B) (1), and the ESTS guidelines describe that the choice between mediastinoscopy with biopsies, or with pre-surgical lymphadenectomies (VAMLA or TEMLA) or endoscopic staging by EBUS/EUS with FNA depends on local expertise (level of evidence V) (2) (Table 2). Regarding the accuracy of minimal invasive methods for patients with tumours classified as cN1 on PET-CT, Yasufuku et al. (35) report a sensibility of 0.43, and Dooms et al. (17), based on the results from a recent prospective multicentre study (ASTER II) reported a sensitivity of only 0.38 for endosonography to detect N2 disease. This sensitivity increased to 0.73 by adding a confirmatory mediastinoscopy to validate negative endosonographies (17). Recently, Decaluwé et al. (18) have reported the results from the first prospective multicentre study (ASTER III) to evaluate the performance of surgical mediastinal staging (by mediastinoscopy or by VAMLA) in patients with cN1 obtaining a global sensitivity of 0.73 and a NPV of 0.92 with a prevalence of 26%. Regarding those patients staged by VAMLA (31%), it is important to point out its excellent results: no complications and no false negative results. Based on the results from ASTER II and III, the authors concluded that the preferred technique for patients with tumours classified as cN1 should be surgical methods such as mediastinoscopy or VAMLA (Table 2).
Finally, there is another subgroup of patients considered as intermediate risk of N2 disease according on the revised ESTS guidelines: those with high SUVmax, cN0 but size greater than 3 cm and especially in adenocarcinomas. Invasive staging would be indicated in this case (2). Again, the decision on the best technique to start with would depend on local expertise to adhere to minimal requirements for staging (2) (Table 2). In the literature, the rate of unsuspected N2 disease for tumours classified as cN0 and tumour size >3 cm is: 6–14.8% (23,24). Recently, Call et al. (5) reported a rate of 22.2% (19% N2 tumours and 3.2% N3 tumours) based on the results from a prospective study to validate the feasibility and accuracy of VAMLA as a method for pre-surgical mediastinal staging. A comparative well-designed study between endosonography methods and surgical techniques should be necessary to analyse which is the best staging procedure for this subgroup of patients in terms of performance. Meanwhile, it is recommendable to validate negative results of endosonographies with a surgical procedure in the same line of those patients with tumours classified as cN1.
Systematics and requirements for mediastinal surgical staging
Mediastinoscopy for lung cancer staging should be thorough, but the thoroughness of the exploration depends on its indication. If mediastinoscopy is indicated to confirm N3 disease, and confirmation is obtained by intraoperative frozen section, there is no need to continue the exploration unless the patient is in a protocol that requires more information. However, when there are no clear signs of mediastinal disease on CT or PET, mediastinoscopy should be systematic and complete. It is better to start by exploring and obtaining biopsy of the contralateral paratracheal nodes to rule out or confirm N3 disease, and then to proceed with the subcarinal nodes, and finally with the ipsilateral paratracheal nodes (36).
Ideally, five nodal stations (2R, 2L, 4R, 4L and 7) should be examined routinely (1,2). The ESTS guidelines determine that, at least, the following nodal stations should be explored and biopsied: right and left inferior paratracheal lymph nodes (stations 4R and 4L) and subcarinal lymph nodes (station 7) (2). It is also recommended, when required to determine subsequent treatment strategy, to biopsy station 10R (below the azygos vein) and station 10L (below the upper rim of the left pulmonary artery) (2). For cancers of the left lung, exploration of the subaortic and para-aortic nodes is also required. The biopsy of the lower mediastinal lymph nodes (stations 8 and 9) should be performed if it changes the treatment strategy, especially when extracapsular nodal disease is suspected (2).
Mediastinoscopy does not reach the aortopulmonary window (stations 5 and 6) and stations 8 and 9. When invasive staging of these stations is required, one of the following surgical techniques (described in sections below) can be added: parasternal mediastinotomy, ECM or video-assisted thoracoscopic surgery (VATS).
Other indications
Any mediastinal lesion (tumour, cyst, inflammation, infection or fibrosis) located within the range of the exploration can be diagnosed by mediastinoscopy. Moreover, this procedure can be used as a therapeutic tool such as closure of post-pneumonectomy bronchopleural fistula, removal of mediastinal cysts or parathyroid tumours, among others (37). In addition to its main indication, the lung cancer staging (topic extensively discussed in the previous sections), mediastinoscopy is also useful for the staging of other thoracic malignancies. The ESTS, the European Respiratory Society, and the American Society of Clinical Oncology, in their clinical practice guidelines for the treatment of malignant pleural mesothelioma recommend invasive staging of the mediastinum with EBUS or mediastinoscopy to select patients for radical resection or multimodality treatment (38,39). Likewise, in patients with lung metastases, mediastinal lymph node involvement is also an independent negative prognostic factor for survival after metastasectomy (40-43). Thus, mediastinoscopy may be useful to select potential candidates for pulmonary resection.
Results
The reliability of mediastinoscopy depends on its thoroughness, based on the number of biopsies performed and the number of nodal stations explored (44). This accounts for the important heterogeneity in the reported sensitivity and NPV that ranges from 0.32 to 0.97 (median of 0.78) and 0.8 to 0.99 (median of 0.91), respectively (1) (Table 1). In addition to the number of lymph nodes explored and biopsied, the use of a video mediastinoscope could also influence the accuracy of this technique. Videomediastinoscopy (VAM) provides better visualization of the operative field than conventional mediastinoscopy (CM) and facilitates the teaching process. Although some authors found an increase in the number of LN or LN stations biopsied, no difference in sensitivity or NPV was found in favour of VAM (45). However, in the ACCP systematic review, in pooling the data from 995 VAMs, the median sensitivity was higher (0.89) in comparison with the median sensitivity of 9,267 conventional mediastinoscopies (0.78) (1) (Table 1).
Other surgical staging procedures
Parasternal mediastinotomy
Anterior mediastinotomy was described by Stemmer et al. (46) and McNeill and Chamberlain (47) in 1965 and 1966, respectively. This procedure consists of a left parasternal incision at the level of the second or third intercostal space, to access the subaortic and the para-aortic nodal stations (stations 5 and 6), that cervical mediastinoscopy cannot reach. Thus, this is the main indication of this procedure, mediastinal staging of bronchogenic carcinoma of the left lung in accordance with the recommendations of ESTS guidelines (2) (Table 2). Since this technique can be performed on the right side and it also allows the exploration of the pleural space, the hilum, the lung parenchyma and the pericardium if needed, the following indications have been described: (I) completion of lung cancer staging process: diagnosis of pleural or pericardial effusions, additional nodules in the lung and exploration of tumours contacting or invading the mediastinum or vascular structures such as superior vena cava or the aortic arch; (II) diagnosis of anterior mediastinal tumours or inflammations (lymphoma, sarcoidosis, thymic tumours).
For the staging of lung cancer, the median sensitivity and NPV reported are 0.71 and 0.91, respectively (1) (Table 1).
ECM
ECM, a technique described by Specht in 1965 (48) and popularized by Ginsberg (49) years later for staging carcinomas of the left lung, can be a good alternative to the classic parasternal mediastinotomy because it allows the assessment of para-aortic and subaortic nodal stations (stations 5 and 6) through the same incision of the standard cervical mediastinoscopy (Table 2). Apart from its main indication, the assessment of the aortopulmonary window in patients with bronchogenic carcinoma of the left lung, this procedure is an excellent tool to obtain a biopsy of undiagnosed anterior mediastinal tumours or lymph nodes that have not been diagnosed by other methods such as transthoracic needle aspiration or Tru-Cut. Similarly, as parasternal mediastinotomy, this approach can be used to enter the pleural space (mediastino-pleuroscopy) to assess the primary tumour, pleural effusion, lung nodules, parietal pleural nodules and diaphragmatic and pericardial lesions (49,50).
Regarding its results in the staging of left lung cancers, a median sensitivity of 0.71 and NPV of 0.91 have been described (1) (Table 1). Focusing on the sensitivity of this procedure, when ECM is performed selectively according to the results of CT and PET, the sensitivity increases (51). This could probably be explained by the higher prevalence of N2–3 disease in patients with enlarged lymph nodes or abnormal uptake in PET.
VATS
VATS for the staging of lung cancer, can be useful for the assessment of the T, the N and the M descriptors (Table 2). VATS allows the surgeon to biopsy or resect peripheral lung tumours, providing diagnosis and staging information in the following clinical scenarios: (I) synchronous lung cancer; (II) T3 [separate tumour nodule(s) in the same lobe]; (III) T4 [separate tumour nodule(s) in a different ipsilateral lobe]; (IV) M1a [separate tumour nodule(s) in the contralateral lung] (52). In relation to its role in the diagnosis of pleural or pericardial effusion, VATS achieves a definitive diagnosis in the 90–95% of cases (53).
Regarding its indication for mediastinal staging, on the right side, VATS allows the assessment of 10R, 4R, 7, 8R and 9R nodal stations. On the left side, it allows the assessment of 10L, 5, 6, 7, 8L and 9L nodal stations, remaining unexplored the left paratracheal nodes (4L station) due to its difficult access. Although VATS is limited to the assessment of only one side of the mediastinum, it is a procedure to have in mind as an alternative to parasternal mediastinotomy and ECM for exploring the aortopulmonary window. Staging values of VATS show a sensitivity ranging from 0.58–1 (median 0.99) and a false negative rate of 4% (1) (Table 1).
Transcervical lymphadenectomies
During the last decade, two new surgical staging procedures were developed allowing a step forward beyond classic mediastinoscopy: VAMLA and TEMLA. The main difference between these procedures is that VAMLA is an endoscopic technique performed through a video mediastinoscope, and TEMLA is an open procedure assisted by a video mediastinoscope or a videothoracoscope, depending on the nodal stations dissected. Their range of exploration also differs: with TEMLA all mediastinal nodal stations from supraclavicular to para-oesophageal can be explored (stations 1, 2R, 2L, 4R, 4L, 7, 8, 3a, 3p, 10L, 10R, 5, and 6), whereas VAMLA explores the right and left paratracheal, subcarinal and hilar nodes (stations 2R, 2L, 4R, 4L, 7, 10R and 10L). The aim of both procedures is the complete clearance of the all mediastinal nodal stations explored (this includes lymph nodes and surrounding adipose tissue), allowing the identification of minimal nodal disease that is not identified on CT or PET. Therefore, the ideal indication for transcervical lymphadenectomies is in tumours without suspicion of N2–3 by CT and PET. In accordance with this premise, the following indications have been described: central tumours, cN1 tumours, left-sided tumours, bilateral synchronous lung cancer and preresectional lymphadenectomy in video-assisted thoracoscopic lobectomy. Regarding the accuracy of these procedures, high sensitivity and accuracy have been reported (5-8,30): 0.88–0.96 and 0.94–0.99, respectively, and, as mentioned in the previous section, these represent the best staging values reported to date for those patients without suspicion of N2 by CT and PET (Tables 1,2).
Role of surgical exploration of the mediastinum in restaging
The assessment of an objective response after induction therapy continues to be a diagnostic challenge. For this reason, the use of mediastinal downstaging as a criterion to select patients for surgery requires a reliable restaging method to predict pathologic stage before lung resection. The ESTS guidelines for preoperative lymph node staging for NSCLC recommend histological confirmation of objective response after induction therapy. This confirmation can be done with endosonography techniques. However, the use of an invasive surgical technique is still recommended when the results of endoscopic procedures are negative (2).
Mediastinoscopy
Mediastinoscopy in restaging can be performed in the following situations: (I) after induction therapy with no pretherapeutic invasive diagnosis; (II) after induction therapy with mediastinal histological confirmation by endoscopic techniques; (III) after induction therapy preceded by staging mediastinoscopy. In this case, mediastinoscopy is a reoperation and is named remediastinoscopy (reMS).
The use of a first mediastinoscopy for restaging is addressed in a small series (54). In this article, NPV of 0.90 with a prevalence of pathologic postinduction (yp)N2 of 46% were reported. Theoretically, this approach could be a good strategy to perform an easier and safer mediastinoscopy due to the absence of adhesions in the mediastinum.
reMS does not differ much from a CM. However, reMS is technically more demanding because of peritracheal adhesions, resulting in a lower accuracy in comparison with the first procedure. The main goal of this procedure is to take new biopsies of those nodes that had been positive at first mediastinoscopy. Moreover, if it is technically feasible, other nodal stations should be reached to rule out subclinical progression of the disease. Although reMS is not a common procedure, several authors have reported its feasibility and the following results for sensitivity and NPV: 0.61–0.71 and 0.73–0.86, respectively (55-58).
Transcervical lymphadenectomies
After a properly performed transcervical lymphadenectomy, the restaging of the mediastinum is unnecessary because there is no material left for a new biopsy. Thus, for primary staging, VAMLA and TEMLA represent a new paradigm. Firstly, transcervical lymphadenectomies could also be considered part of the induction treatment because the mediastinum is staged and downstaged by these operations. Secondly, due to the fact that nodal restaging is unnecessary, new parameters should be used to select patients for lung resection after induction, such as the stability of the primary tumour and the absence of extrathoracic disease based on the results of postinduction CT or PET. Finally, intraoperative pathologic study of the remaining lymph nodes should confirm the absence of nodal involvement before proceeding with lung resection, especially if pneumonectomy is required.
Focusing on the use of these procedures for restaging after induction therapy, only TEMLA has been validated on two retrospective studies conducted in the same institution. In the first series with 63 patients, the diagnosis of N2–3 disease before induction treatment was confirmed with invasive techniques in 27 patients (20 with endosonography and 7 with mediastinoscopy), and with CT in 36. Sensitivity and NPV of restaging TEMLA were 0.95, and 0.97, respectively (59). In the second series with 176 patients treated with chemo or chemoradiotherapy, the restaging values of EBUS and/or EUS (88 patients) were compared with those of TEMLA (78 patients). There was a significant difference between EBUS/EUS and TEMLA for sensitivity (0.64 and 1; P<0.01) and NPV (0.82 and 1; P<0.01) in favour of TEMLA (60).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211-50S.
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Carlens E. Mediastinoscopy: a method for inspection and tissue biopsy in the superior mediastinum. Dis Chest 1959;36:343-52. [Crossref] [PubMed]
- Tournoy KG, Keller SM, Annema JT. Mediastinal staging of lung cancer: novel concepts. Lancet Oncol 2012;13:e221-9. [Crossref] [PubMed]
- Call S, Obiols C, Rami-Porta R, et al. Video-assisted mediastinoscopic lymphadenectomy for staging non-small cell lung cancer. Ann Thorac Surg 2016;101:1326-33. [Crossref] [PubMed]
- Witte B, Wolf M, Huertgen M, et al. Video-assisted mediastinoscopic surgery: clinical feasibility and accuracy of mediastinal lymph node staging. Ann Thorac Surg 2006;82:1821-7. [Crossref] [PubMed]
- Turna A, Demirkaya A, Ozkul S, et al. Video-assisted mediastinoscopic lymphadenectomy is associated with better survival than mediastinoscopy in patients with resected non-small cell lung cancer. J Thorac Cardiovasc Surg 2013;146:774-80. [Crossref] [PubMed]
- Zielinski M. Transcervical extended mediastinal lymphadenectomy (TEMLA): the standard procedure and its variations. In: Zielinski M, Rami-Porta R. editors. The transcervical approach in thoracic surgery. Heidelberg: Springer, 2014:101-16.
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project. A proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- De Leyn P, Lardinois D, Van Schil PE, et al. ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur J Cardiothorac Surg 2007;32:1-8. [Crossref] [PubMed]
- Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA 2010;304:2245-52. [Crossref] [PubMed]
- Um SW, Kim HK, Jung SH, et al. Endobronchial ultrasound versus mediastinoscopy for mediastinal nodal staging of non-small cell lung cancer. J Thorac Oncol 2015;10:331-7. [Crossref] [PubMed]
- Korevaar DA, Crombag LM, Cohen JF, et al. Added value of combined endobronchial and oesophageal endosconography for mediastinal nodal staging in lung cancer: a systematic review and meta-analysis. Lancet Respir Med 2016;4:960-8. [Crossref] [PubMed]
- Ye B, Cheng M, Li W, et al. Predictive factors for lymph node metastasis in clinical stage IA lung adenocarcinoma. Ann Thorac Surg 2014;98:217-23. [Crossref] [PubMed]
- Koike T, Koike T, Yamato Y, et al. Predictive risk factors for mediastinal lymph node metastasis in clinical stage IA non-small-cell lung cancer patients. J Thorac Oncol 2012;7:1246-51. [Crossref] [PubMed]
- Obiols C, Call S. Pros: should a patient with stage IA non-small cell lung cancer undergo invasive mediastinal staging? Transl Lung Cancer Res 2016;5:247-50. [Crossref] [PubMed]
- Dooms C, Tournoy KG, Schuurbiers O, et al. Endosonography for mediastinal nodal staging of clinical N1 non-small cell lung cancer: a prospective multicenter study. Chest 2015;147:209-15. [Crossref] [PubMed]
- Decaluwé H, Dooms C, D'Journo XB, et al. Mediastinal staging by videomediastinoscopy in clinical N1 non-small cell lung cancer: a prospective multicentre study. Eur Respir J 2017;50. [Crossref] [PubMed]
- Yendamuri S, Battoo A, Dy G, et al. Transcervical extended mediastinal lymphadenectomy: experience from a North American Cancer Center. Ann Thorac Surg 2017;104:1644-9. [Crossref] [PubMed]
- Witte B, Messerschmidt A, Hillebrand H, et al. Combined videothoracoscopic and videomediastinoscopic approach improves radicality of minimally invasive mediastinal lymphadenectomy for early stage lung carcinoma. Eur J Cardiothorac Surg 2009;35:343-7. [Crossref] [PubMed]
- Kim HJ, Kim YH, Choi SH, et al. Video-assisted mediastinoscopic lymphadenectomy combined with minimally invasive pulmonary resection for left-sided lung cancer: feasibility and clinical impacts on surgical outcomes. Eur J Cardiothorac Surg 2016;49:308-13. [Crossref] [PubMed]
- Zieliński M, Rybak M, Solarczyk-Bombik K, et al. Uniportal transcervical video-assisted thoracoscopic surgery (VATS) approach for pulmonary lobectomy combined with transcervical extended mediastinal lymphadenectomy (TEMLA). J Thorac Dis 2017;9:878-84. [Crossref] [PubMed]
- Wang J, Welch K, Wang L, et al. Negative predictive value of positron emission tomography and computed tomography for stage T1-2N0 non-small-cell lung cancer: a meta-analysis. Clin Lung Cancer 2012;13:81-9. [Crossref] [PubMed]
- Gómez-Caro A, Boada M, Cabañas M, et al. False-negative rate after positron emission tomography/ computer tomography scan for mediastinal staging in cI stage non-small-cell lung cancer. Eur J Cardiothorac Surg 2012;42:93-100. [Crossref] [PubMed]
- Shingyoji M, Nakajima T, Yoshino M, et al. Endobronchial ultrasonography for positron emission tomography and computed tomography-negative lymph node staging in non-small cell lung cancer. Ann Thorac Surg 2014;98:1762-7. [Crossref] [PubMed]
- Ong P, Grosu H, Eapen GA, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for systematic nodal staging of lung cancer in patients with N0 disease by computed tomography and integrated positron emission tomography-computed tomography. Ann Am Thorac Soc 2015;12:415-9. [Crossref] [PubMed]
- Vial MR, O’Connell OJ, Grosu HB, et al. Diagnostic performance of endobronchial ultrasound-guided mediastinal lymph node sampling in early stage non-small cell lung cancer: A prospective study. Respirology 2018;23:76-81. [Crossref] [PubMed]
- Naur TMH, Konge L, Clementsen PF. Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration for Staging of Patients with Non-Small Cell Lung Cancer without Mediastinal Involvement at Positron Emission Tomography-Computed Tomography. Respiration 2017;94:279-84. [Crossref] [PubMed]
- Detterbeck FC, Jantz MA, Wallace M, et al, American College of Chest Physicians. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:202–20S.
- Zieliński M, Hauer L, Hauer J, et al. Transcervical extended mediastinal lymphadenectomy (TEMLA) for staging of non-small-cell lung cancer (NSCLC). Pneumonol Alergol Pol 2011;79:196-206. [PubMed]
- Obiols C, Call S, Rami-Porta R, et al. Survival of patients with unsuspected pN2 non-small cell lung cancer after an accurate preoperative mediastinal staging. Ann Thorac Surg 2014;97:957-64. [Crossref] [PubMed]
- Decaluwé H, Dooms C. Cons: should a patient with stage IA non-small cell lung cancer undergo invasive mediastinal staging? Transl Lung Cancer Res 2016;5:251-3. [Crossref] [PubMed]
- Lee PC, Port JL, Korst RJ, et al. Risk factors for occult mediastinal metastases in clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:177-81. [Crossref] [PubMed]
- Turna A, Melek H, Kara HV, et al. Validity of the updated European Society of Thoracic Surgeons staging guideline in lung cancer patients. J Thorac Cardiovasc Surg 2018;155:789-95. [Crossref] [PubMed]
- Yasufuku K, Nakajima T, Waddell T, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for differentiating N0 versus N1 lung cancer. Ann Thorac Surg 2013;96:1756-60. [Crossref] [PubMed]
- Rami-Porta R, Call S. Invasive staging of mediastinal lymph nodes: mediastinoscopy and remediastinoscopy. Thorac Surg Clin 2012;22:177-89. [Crossref] [PubMed]
- Rami-Porta R, Call S, Serra-Mitjans M. Mediastinoscopy. In: Zielinski M, Rami-Porta R. editors. The transcervical approach in thoracic surgery. Heidelberg: Springer, 2014:9-27.
- Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479-95. [Crossref] [PubMed]
- Kindler HL, Ismaila N, Armato SG 3rd, et al. Treatment of malignant pleural mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1343-73. [Crossref] [PubMed]
- Pfannschmidt J, Klode J, Muley T, et al. Nodal involvement at the time of pulmonary metastasectomy: experiences in 245 patients. Ann Thorac Surg 2006;81:448-54. [Crossref] [PubMed]
- García-Yuste M, Cassivi S, Paleru C. Thoracic lymphatic involvement in patients having pulmonary metastasectomy: incidence and the effect on prognosis. J Thorac Oncol 2010;5:S166-9. [Crossref] [PubMed]
- Embun R, Rivas de Andrés JJ, Call S, et al. Causal model of survival after pulmonary metastasectomy of colorectal cancer: a nationwide prospective registry. Ann Thorac Surg 2016;101:1883-90. [Crossref] [PubMed]
- Call S, Rami-Porta R, Embún R, et al. Impact of inappropriate lymphadenectomy on lung metastasectomy for patients with metastatic colorectal cancer. Surg Today 2016;46:471-8. [Crossref] [PubMed]
- Detterbeck F, Puchalski J, Rubinowitz A, et al. Classification of the thoroughness of mediastinal staging of lung cancer. Chest 2010;137:436-42. [Crossref] [PubMed]
- Zakkar M, Tan C, Hunt I. Is video mediastinoscopy a safer and more effective procedure than conventional mediastinoscopy? Interact CardioVasc Thorac Surg 2012;14:81-4. [Crossref] [PubMed]
- Stemmer EA, Calvin JW, Chandor SB, et al. Mediastinal biopsy for indeterminate pulmonary and mediastinal lesions. J Thorac Cardiovasc Surg 1965;49:405-11. [PubMed]
- McNeill TM, Chamberlain JM. Diagnostic anterior mediastinoscopy. Ann Thorac Surg 1966;2:532-9. [Crossref] [PubMed]
- Specht G. Erweiterte Mediastinoskopie. Thoraxchir Vask Chir 1965;13:401-7. [PubMed]
- Ginsberg RJ, Rice TW, Goldberg M, et al. Extended cervical mediastinoscopy. A single staging procedure for bronchogenic carcinoma of the left upper lobe. J Thorac Cardiovasc Surg 1987;94:673-8. [PubMed]
- Deslauriers J, Beaulieu M, Dufour C, et al. Mediastinopleuroscopy: a new approach to the diagnosis of intrathoracic diseases. Ann Thorac Surg 1976;22:265-9. [Crossref] [PubMed]
- Obiols C, Call S, Rami-Porta R, et al. Extended cervical mediastinoscopy: mature results of a clinical protocol for staging bronchogenic carcinoma of the left lung. Eur J Cardiothorac Surg 2012;41:1043-6. [Crossref] [PubMed]
- Detterbeck FC, Nicholson AG, Franklin WA, et al. The IASLC Lung Cancer Staging Project: Summary of proposals for revisions of the classification of lung cancers with multiple pulmonary sites of involvement in the forthcoming eight edition of the TNM classification. J Thorac Oncol 2016;11:639-50. [Crossref] [PubMed]
- Krasna MJ. The role of thoracoscopy in the management of cancer patients. Semin Oncol 2008;35:129-33. [Crossref] [PubMed]
- Lardinois D, Schallberger A, Betticher D, et al. Postinduction video-mediastinoscopy is as accurate and safe as video-mediastinoscopy in patients without pretreatment for potentially operable non-small cell lung cancer. Ann Thorac Surg 2003;75:1102-6. [Crossref] [PubMed]
- Stamatis G, Fechner S, Hillejan L, et al. Repeat mediastinoscopy as a restaging procedure. Pneumologie 2005;59:862-6. [Crossref] [PubMed]
- Marra A, Hillejan L, Fechner S, et al. Remediastinoscopy in restaging of lung cancer after induction therapy. J Thorac Cardiovasc Surg 2008;135:843-9. [Crossref] [PubMed]
- De Waele M, Serra-Mitjans M, Hendriks J, et al. Accuracy and survival of repeat mediastinoscopy after induction therapy for non-small cell lung cancer in a combined series of 104 patients. Eur J Cardiothorac Surg 2008;33:824-8. [Crossref] [PubMed]
- Call S, Rami-Porta R, Obiols C, et al. Repeat mediastinoscopy in all its indications: experience with 96 patients and 101 procedures. Eur J Cardiothorac Surg 2011;39:1022-7. [Crossref] [PubMed]
- Zieliński M, Hauer L, Hauer J, et al. Non-small-cell lung cancer restaging with transcervical extended mediastinal lymphadenectomy. Eur J Cardiothorac Surg 2010;37:776-80. [Crossref] [PubMed]
- Zielinski M, Szlubowski A, Kołodziej M, et al. Comparison of endobronchial ultrasound and/or endoesophageal ultrasound with transcervical extended mediastinal lymphadenectomy for staging and restaging of non-small-cell lung cancer. J Thorac Oncol 2013;8:630-6. [Crossref] [PubMed]


