The diagnosis of coronary plaque stability by multi-slice computed tomography coronary angiography
Introduction
Coronary artery disease (CAD) remains a leading cause of morbidity and mortality worldwide despite some major pharmacologic and risk stratification advancement over the last two decades (1,2). In clinical practice, early detection of culprit plaque in advance with coronary computed tomographic angiography (CCTA), coronary angiography (CAG), or intravascular ultrasound (IVUS) may enable early intervention to prevent acute coronary syndrome (ACS) or other plaque-related diseases, decrease morbidity and mortality rates, and improve the quality of life for patients with CAD (3-8). While CAG is an invasive method and more accurate in evaluating the degree of coronary artery stenosis, and although IVUS is the gold standard for the diagnosis of plaque characteristics, it is limited by high cost and invasive nature of the test. CCTA is a robust non-invasive method to assess CAD (9-12) and is an important non-invasive method to analyze the plaque compositions, especially for the non-calcified plaques (13). Prior studies had put an emphasis on the correlation between CCTA and CAG, or between CCTA and IVUS, in the discrimination of the plaque characterizations, while some only revealed one point of the culprit plaque characteristics (14-20).
The study is to investigate the differential characteristics of the non-calcified unstable coronary plaques and the stable coronary plaques using noninvasive multi-slice computed tomography (MSCT) with a comparison to CAG and/or IVUS.
Methods
Patients
We collected 281 cases from the department of cardiology, Zhongshan hospital, Fudan university, which is a tertiary referral hospital. And according to the strict group entry standards and exclusion standards, we finally enrolled 60 patients with confirmed unstable plaques and stable plaques by CAG and/or IVUS. Forty patients underwent IVUS and CAG, and 20 patients with ruptured plaques and unstable plaques were confirmed by CAG. All patients were divided into two groups: the group of unstable plaques including 34 patients with 37 plaques (32 male, 2 female; age of 42–74 years old with a mean age of 63), of which one patient had two plaques and the other one had three plaques; the group of stable plaques including 26 patients with 31 plaques (17 male, 9 female; age of 43–77 years old with a mean age of 61), and 5 of them had two plaques.
Thirty-seven cases had hypertension (23 from unstable group, 14 from stable group), 21 cases had diabetes mellitus (14 from unstable group, 7 from stable group), 24 cases had a history of smoke (17 from unstable group, 7 from stable group). Forty-four patients had unstable angina, including 34 from unstable group, and 10 from stable group. The 10 cases in stable group with unstable angina, did have unstable plaques in other vessel segments, but were excluded because of large area of calcification. Twenty cases from stable group had stable angina.
The entry standards of our study. For the unstable group, all the three conditions must be met at the same time. First, all the patients had undergone CAG and/or IVUS, and CCTA. Second, all the plaques were diagnosed as unstable plaques or ruptured plaques by CAG and/or IVUS. Third, all the plaques had no large area of calcification, which would influence the plaque evaluation. For the stable group, all the three conditions must be met at the same time. First, all the patients had undergone CAG and IVUS, and CCTA. Second, all the plaques were diagnosed as stable plaques by IVUS. Third, all the plaques had no large area of calcification, which would influence the plaque observation.
The exclusion standards of our study. Cases with one such condition as below were excluded. First, the patients had undergone PCI surgery and the vessel segment we were interested had coronary stent. Second, the patients had undergone coronary artery bypass surgery. Third, the vessel segment had so many calcified plaques that we could not evaluate the plaque characteristics accurately.
In the group of unstable plaques, 6 patients with ruptured plaque and 8 patients with unstable plaque or lipid plaque were diagnosed by IVUS. While 14 patients with ruptured plaque and another 6 patients with unstable plaque were only diagnosed by CAG, and thrombus formation after repeated aspiration was observed in the vessel segments of the 6 unstable plaques. Then, the 20 patients were also classified as the unstable plaque group. In the group of stable plaques, all 26 patients with fibrous plaque were diagnosed by IVUS, and they had no plaque with signs of instability.
In 36 patients (24 from the unstable group, 12 from the stable group), CAG and/or IVUS were performed no longer than two weeks after coronary CTA. In 2 cases (from the stable plaques), CAG and/or IVUS were performed within two weeks before coronary CTA. Fifteen (5 from unstable group, 10 from stable group) were performed within 4 weeks, 2 (from stable group) were performed within 3 months, 3 (from stable group) were performed within 12 months, 1 (from unstable group) was performed within 14 months, and the rest one (from unstable group) was performed within 15 months.
CT scanning and data acquisition
All sixty patients underwent CCTA. CCTA datasets were acquired using a 320-row volumetric scanner (Aquilion ONE, Toshiba Medical System, Japan) for 8 patients and a Dual Source CT scanner (SOMATOM Definition Flash, Siemens medical system, Germany) for 52 patients. The tube voltage of 120 kV and the intelligent mAs were employed by both scanners. The other imaging parameters were as follow: 0.33 s/r tube rotation speed, 0.2–0.43 pitch and 7–11 s scan time (SOMATOM Definition Flash) and 0.35–0.45 s/r tube rotation speed and 1–3 cardiac cycle scan time (Aquilion ONE).
Non-ionic contrast agents Iopamidol 370 (Iopamidol, Bracco, Milan, Italy) or Ultravist 370 (Bayer Schering Pharma AG, Berlin, Germany) with an amount of 60–70 mL followed by a saline flush of 20 mL were administered via antecubital vein using automated injection system (Vistron CT Injection System, Medrad, USA) at a flow rate of 5 mL/s. The trigger threshold was set at 120 HU (SOMATOM Definition Flash) or 300 HU (Aquilion ONE) within the ascending aorta. The CT value of contrast media within the lumen of plaque region was 238–641 HU with an average of 406.5±79.6 HU.
Images with their most optimal phase were sent to Advantage Workstation Volume Share 2 (GE Healthcare, Milwaukee, USA) for reformations such as volume rendering (VR), curved planar reformation (CPR), multi-planar reformation (MPR) and maximum intensity projection (MIP).
Image evaluation
All images were evaluated with a uniform window width and window level (1,120 HU, 300 HU). The napkin-ring sign was defined as a peripheral high-density ring enveloping a low-density patch on CCTA and its CT value less than 130 HU (14). The CT value of the non-calcified plaques was measured on an orthogonal cross section reformatted image of the plaque region. A region of interest (ROI) was precisely placed within the central area of the plaque, and the napkin ring area was spared. In terms of measurable napkin-ring sign, the section corresponding to the thinnest napkin ring on CCTA was chosen to measure ring’s thickness and CT value within the plaque center. The degree of coronary artery stenosis at the same section was also documented.
Two experienced radiologists, who were blind to all the clinical data, were recruited to jointly identify the plaque vulnerability by the napkin-ring sign, plaque CT value, thickness of napkin ring, and degree of coronary artery stenosis. The first two were the primary diagnostic indexes, and the remaining two were the secondary diagnostic indexes.
The reason we enrolled 20 cases confirmed by CAG
We included 20 cases (14 ruptured plaques and 6 unstable plaques) confirmed by CAG, Since IVUS is the gold standard of plaque characteristic. In this study, 40 patients underwent both CAG and IVUS. Six of them with ruptured plaques were diagnosed, while the remaining 34 patients with no ruptured plaques. In our study there was a perfect consensus between CAG and IVUS for identifying plaque rupture (P<0.01, r=1.00). We included 14 ruptured plaques confirmed by CAG, because we believed that a positive CAG result would be matched with a positive IVUS result. But it does not mean that CAG can be used perfectly to diagnose plaque rupture, as CAG can provide limited information about the composition of the vessel wall.
In other 6 cases with unstable plaques identified by CAG, thrombus formation was observed in the vessel segments of the plaque region.
Statistical analysis
All data were statistically analyzed using Stata software package version 10.0 (StataCorp LP, College Station, Texas, USA) or IBM SPSS version 22.0 (IBM, USA). Comparison of the thickness of measurable napkin rings was performed using the Mann-Whitney U test. The Chi-square test was applied to compare the differences of the napkin-ring sign, plaque distribution and degree of lumen stenosis of the plaque region. Comparison of plaque attenuation between the two groups was carried out using independent sample t test. The differences of the plaque characteristics between MSCT and IVUS were analyzed by Wilcoxon matched-pairs signed ranks test. The correlation between MSCT and IVUS was performed using rank correlation.
Based on the measurable napkin rings, the napkin ring thickness, plaque CT value and degree of coronary artery stenosis were performed using multivariate logistic regression analysis. Additionally, the napkin-ring sign, plaque CT value and degree of coronary artery stenosis were also analyzed by multivariate logistic regression analysis. The statistical method was reported previously (21). Briefly, a logistic regression equation was formed by multivariate regression analysis, and the exact pair-matched data of the napkin-ring sign and plaque CT value of the 68 plaques were backtested via the equation to compute exact probabilities. Then, by the receiver operating characteristic (ROC) curve analysis and Youden index, the ultimate optimal combined cutoff values of the napkin-ring sign and plaque CT value were identified to predict unstable plaques. The difference was statistically significant if P<0.05.
Statement of ethics approval
The ethics approval of our study was waivered by the institutional review board of our hospital, because it was a retrospective study, and we did not need to give the participants informed consent before taking part. The ethics committee of Zhongshan Hospital, Fudan university, also approved our investigation with a waiver of informed consent.
Results
The napkin ring thickness
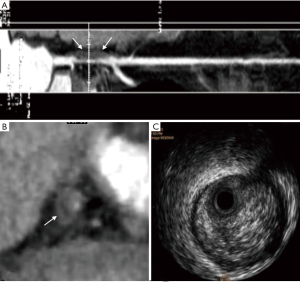
The measurable thickness of napkin ring showed the napkin ring of 32 unstable plaques was thinner than that of 6 stable plaques (P<0.001). The median napkin-ring thickness of unstable plaques was 0.6 mm with a range from 0.2 to 1.0 mm (Figure 1), while the median napkin-ring thickness of stable plaques was 1.0 mm with a range from 0.8 to 1.7 mm (Figure 2).
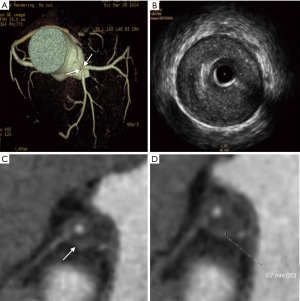

The napkin-ring sign
As shown in Table 1, the napkin-ring sign in the unstable group was more frequently observed than that of the stable group (P<0.05). The napkin-ring sign was found in 33 plaques (89.2%) in the unstable group (Figure 1), and the sign was only observed in 7 plaques (22.6%) in stable group (Figures 2,S1). The plaques without napkin-ring sign appeared as uniform low-density.

Full table
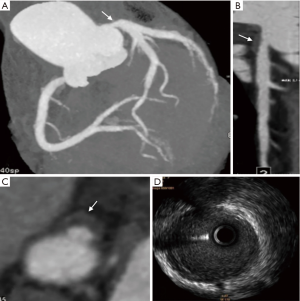
The plaque CT values
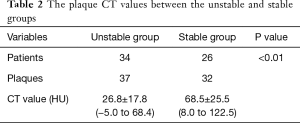
The plaque CT value of the stable and unstable groups was summarized in Table 2. The mean plaque attenuation of unstable plaques was 26.8±17.8 HU with a range from −5 to 68.4 HU, and that of stable plaques was 68.5±25.5 HU with a range from 8.0 to 122.5 HU. The density of unstable plaques (Figure 3) was lower than that of stable group (Figure 4) (P<0.05), although there was some overlap on the plaque CT value from the two groups.
Full table

The degree of coronary artery stenosis
The plaques within the two groups were mainly located in the left anterior descending branch (Table S1) [More Tables were shown than can be included in the article. The interested reader can find them in a supplementary appendix online.]. The difference between the two groups was not statistically significant (P>0.05). Based on CAG, the severity of the lumen stenosis in the two groups were recorded as no stenosis (0%), mild stenosis (<50%), moderate stenosis (75–50%), severe stenosis (95–75%) and occlusion (>95%). As shown in Table 3, the severe stenosis or occlusion rate (70.3%) in the unstable group was higher than that in the stable group (41.9%, P<0.05). Only 2 cases (5.9%) were recorded as occlusion in unstable group.
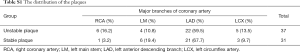
Full table

Full table
The value of multi-slice spiral CT in diagnosing plaque vulnerability
Based on the plaques with measurable napkin ring, the thickness of napkin ring was only one factor to predict unstable plaque (Table 4) and the optimal cutoff value of the thickness is not greater than 0.8 mm, whose sensitivity, specificity, positive predictive value, negative predictive value and accuracy to determine the unstable plaques were 93.8%, 66.7%, 93.8%, 66.7% and 89.5%, respectively. The area under the ROC curve was 0.802 (P<0.001, 95% CI: 0.567–1.000).

Full table
According to the plaques with the napkin sign, the sign and the plaque CT value were great combined factors to predict unstable plaque (Table 5). The optimal combined threshold was the presence of the napkin-ring sign and/or the plaque CT value of less than or equal to 53 HU, whose sensitivity, specificity, positive predictive value, negative predictive value and accuracy to identify unstable plaque were 100%, 58.1%, 74.0%, 100% and 80.9%, respectively. The area under the ROC curve was 0.790 (P<0.001, 95% CI: 0.674–0.907).

Full table
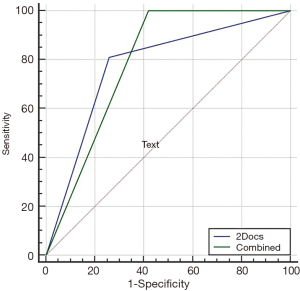
The sensitivity, specificity, positive predictive value, negative predictive value and accuracy of MSCT using the primary and secondary indexes to jointly diagnose unstable plaque by two radiologists were 78.4%, 77.4%, 80.6%, 75.0% and 77.9%, respectively (Table S2) [More Tables were shown than can be included in the article. The interested reader can find them in a supplementary appendix online.]. The area under the ROC curve was 0.779 (P<0.001, 95% CI: 0.678–0.880). By pair-comparison of ROC curves (Figure S2), there was no significant difference to predict unstable plaque between the optimal combined threshold and joint diagnosis by two radiologists (P=0.821, 95% CI: −0.107 to 0.135). This suggested that MSCT had a moderate diagnostic value with subjective evaluation in identifying unstable plaques. There was no statistically significant difference in the diagnosis of plaque vulnerability between MSCT and IVUS (P>0.05), and there was a moderate correlation to determine unstable plaque between MSCT and IVUS (r=0.557, P<0.01).
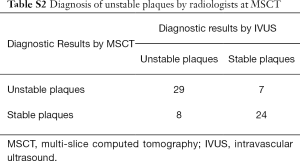
Full table
Discussion
MSCT as a potential role in the diagnosis of unstable plaque
IVUS is the gold standard for the diagnosis of plaque characteristics, but it is limited by high cost and invasive nature of the test. Previous report (22) addressed that CCTA had a good correlation with IVUS in the diagnosis of plaque stability. The present study showed there was no statistically significant difference between CCTA and IVUS in the diagnosis of plaque stability. Compared with IVUS, CCTA has established itself as a non-invasive modality for detecting coronary artery stenosis.
Few paper had systematically identified the imaging characteristics of plaque vulnerability (9). In this study, we identified the plaque stability based on MSCT parameters of the plaque attenuation, napkin-ring sign, thickness of the napkin ring and lumen stenosis. CCTA had a powerful diagnostic value to predict unstable plaques with good accuracies of 89.5% based on the plaques with measurable napkin ring, 80.9% based on the optimal combined threshold of the napkin-ring sign and/or the plaque CT value ≤53 HU, and 77.9% based on jointly diagnosis by two radiologists. We also found that there was no significant difference to predict unstable plaque between the latter two diagnostic performance. It means that CCTA might be a potential diagnostic tool to subjectively or objectively predict unstable plaque for CAD patients in the present study. Moreover, if the napkin-ring sign is present with measurable ring, the thickness of the napkin ring may also have a powerful diagnostic performance to predict vulnerable plaque. All the data suggest that CCTA may have important clinical value in the noninvasive diagnosis of plaque stability, thus suggesting the target patients to get further examination and proper treatment.
The napkin-ring sign
The napkin-ring sign, appearing as a ring-like enhancement on CCTA, is a good biomarker to independent predict culprit lesions for future ACS events in patients with CAD (14). Our investigation showed that the thickness of the napkin ring of vulnerable plaques (median: 0.6 mm) was thinner than that of stable plaques (median: 1.0 mm). The difference was subtle between the two types of plaques, but it was able to discriminate with the naked eye for most patients. Previous studies revealed plaques with a thin fibrous cap was more frequently associated with ACS (23,24) and in vivo evaluation of plaque fibrous cap thickness by intravascular optical coherence tomography and/or IVUS (25-27). Furthermore, Sato et al. (12) found that the presence of ring-like enhancement at CCTA had a higher accuracy of 88% for detection of plaques with a thin fibrous cap in patients with unstable angina pectoris. To the best of our knowledge, the association of the napkin ring thickness between the unstable and stable plaques is little known. Our encouraging results may provide one more promising CT signature for assessing plaque vulnerability by measuring the napkin ring thickness on CCTA image.
In the study, the napkin-ring sign was also found to be able to differentiate unstable plaques (89.2%) from stable plaques (22.6%). To date, the histopathological features of atherosclerotic plaques with napkin-ring sign remains unclear. Maurovich-Horvat et al. (28) suggested that the napkin-ring sign might be caused by the difference in CT value between a lipid-rich necrotic core (i.e., the central low-density area) and fibrous plaque tissue or thin fibrous cap (i.e., the peripheral high-density rim). Other mechanisms such as a central thrombus surrounded by contrast agent, vasa vasorum, hemorrhage, or microcalcification might also lead to a ring-like pattern of vulnerable or ruptured plaques.
As a CT signature of high-risk coronary plaque, the napkin-ring sign was furtherly investigated under the scope of ACS. Pflederer et al. (29) found that a ring-like enhancement on CCTA was seen in 25% of culprit lesions in patients with ACS, but never in patients with stable angina. In a report, ACS was more frequently in plaques with a thin fibrous cap, and 44% of plaques with a thin fibrous cap was detected with a ring-like enhancement on CCTA, but only 4% of plaques without a thin fibrous cap presenting as a ring like enhancement on CCTA (23). Furthermore, Nishio et al. (30) showed that the napkin-ring sign was also able to independently predict ruptured plaques with a high positive predictive value of 88%. These studies suggested that the napkin-ring sign demonstrated on CCTA was strongly associated with future ACS events, which was independent of other high-risk coronary CTA features (14,23,28,31-37). It is indicated that detection of the napkin-ring sign could help identify patients with CAD at high risk of future ACS events. Our study revealed that the napkin-ring sign may discriminate unstable plaques from stable plaques on CCTA, and it also supported these points.
The CT density of the plaques
In the present investigation, the mean plaque attenuation of the unstable plaques (26.8±17.8 HU) was much lower than that of the stable plaques (68.5±25.5 HU), although some overlap might exist between the two groups. This finding was consistent with the results of previous ex vivo coronary artery studies at MSCT (7,38,39) and comparisons between CCTA and IVUS (10-13), which showed that the mean CT density of “lipid-rich” or “soft” plaque was 14–49 HU, and the mean attenuation within “fibrous” or “dense” lesions was between 70 and 104 HU. Pohle et al. (19) showed that the mean CT attenuation within plaques corresponding to hyper-echogenic appearance on IVUS was 121±34 HU and that corresponding to hypo-echogenic appearance was 58±43 HU. Kitagawa et al. (40) revealed that the CT value of plaques with ACS was 24±22 HU, while the CT value of plaques without ACS was 42±29 HU. The variance of plaque CT values might be explained by the following facts: intracoronary attenuation significantly modified the attenuation of plaques assessed with MSCT (38,41); the CT value of plaques was highly dependent on slice width; the inclusion criteria in various studies had no uniform standards; and different coronary plaque-related studies used different CT scanners. Although the CT value between the two types of non-calcified plaques had some certain overlap, the density of unstable plaques was obviously lower than that of stable plaques (13). This might provide an important reference with high accuracy to differentiate unstable plaques from stable plaques in the clinical setting.
In addition, the prevalence of severe lumen stenosis in the present study was much more frequently encountered in unstable plaques (70.3%) than that in stable plaques (41.9%), which might suggest that the plaques with severe lumen stenosis were more likely to be ruptured. These four above-mentioned indexes as important factors to predict unstable plaques contributed unequally, and a further investigation should be conducted in the future. Moreover, our data demonstrated that the plaques of the two groups were mainly distributed in the left anterior descending branch, which was in accordance with previous report (22).
Limitations
This study was a retrospective study. The number of enrolled patients was relatively small. All the patients were diagnosed by IVUS or CAG, however, the CT features could not be precisely compared with pathology. The variation of CT value of the lumen contrast might have an impact on the measurement. Additionally, the positive remodeling of the lumen, which was also an important index to evaluate vulnerable plaques, was not investigated in this study. Finally, with a best effort to minimize the misplacement of ROI for the CT value measurement, the typical plaque area on IVUS might not be well represented on MSCT.
In conclusion, the optimal combined threshold of the napkin-ring sign and/or the plaque CT value ≤53 HU may be a good indicator to predict unstable plaque in patients with CAD. Based on the subgroup of measurable napkin ring, the napkin ring thickness of unstable plaques was thinner than that of stable plaques, and it may also be an independent factor to predict the unstable plaques in patients with CAD.
Acknowledgements
None.
Footnote
Conflicts of Interest: An oral presentation was exhibited by Dr. Feng-xiang Song at the 2017 RSNA Annual Meeting.
Ethical Statement: The ethics approval of our study was waivered by the institutional review board of our hospital, because it was a retrospective study, and we did not need to give the participants informed consent before taking part. The ethics committee of Zhongshan Hospital, Fudan University, also approved our investigation with a waiver of informed consent.
References
- WRITING GROUP MEMBERS, Lloyd-Jones D, Adams RJ, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation 2010;121:e46-e215. [Crossref] [PubMed]
- Kwan AC, Cater G, Vargas J, et al. Beyond coronary stenosis: coronary computed tomographic angiography for the assessment of atherosclerotic plaque burden. Curr Cardiovasc Imaging Rep 2013;6:89-101. [Crossref] [PubMed]
- Hoffmann U, Truong QA, Schoenfeld DA, et al. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med 2012;367:299-308. [Crossref] [PubMed]
- Truong QA, Hayden D, Woodard PK, et al. Sex differences in the effectiveness of early coronary computed tomographic angiography compared with standard emergency department evaluation for acute chest pain: the rule-out myocardial infarction with computer-assisted tomography (ROMICAT)-II Trial. Circulation 2013;127:2494-502. [Crossref] [PubMed]
- Truong QA, Schulman-Marcus J, Zakroysky P, et al. Coronary CT angiography versus standard emergency department evaluation for acute chest pain and diabetic patients: is there benefit with early coronary CT angiography? Results of the randomized comparative effectiveness ROMICAT II Trial. J Am Heart Assoc 2016;5:e003137. [PubMed]
- Tsai IC, Lee T, Lee WL, et al. Use of 40-detector row computed tomography before catheter coronary angiography to select early conservative versus early invasive treatment for patients with low-risk acute coronary syndrome. J Comput Assist Tomogr 2007;31:258-64. [Crossref] [PubMed]
- Brener SJ, Maehara A, Mintz GS, et al. Effect of prior aspirin treatment on patients with acute coronary syndromes: insights from the PROSPECT study. J Invasive Cardiol 2015;27:536-41. [PubMed]
- Dedic A, Lubbers MM, Schaap J, et al. Coronary CT angiography for suspected ACS in the era of high-sensitivity troponins: randomized multicenter study. J Am Coll Cardiol 2016;67:16-26. [Crossref] [PubMed]
- Munnur RK, Cameron JD, Ko BS, et al. Cardiac CT: atherosclerosis to acute coronary syndrome. Cardiovasc Diagn Ther 2014;4:430-48. [PubMed]
- Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015;372:1291-300. [Crossref] [PubMed]
- Min JK, Leipsic J, Pencina MJ, et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA 2012;308:1237-45. [Crossref] [PubMed]
- Sato A, Hoshi T, Kakefuda Y, et al. In vivo evaluation of fibrous cap thickness by optical coherence tomography for positive remodeling and low-attenuation plaques assessed by computed tomography angiography. Int J Cardiol 2015;182:419-25. [Crossref] [PubMed]
- Becker CR, Nikolaou K, Muders M, et al. Ex vivo coronary atherosclerotic plaque characterization with multi-detector-row CT. Eur Radiol 2003;13:2094-8. [Crossref] [PubMed]
- Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 2009;54:49-57. [Crossref] [PubMed]
- Shmilovich H, Cheng VY, Tamarappoo BK, et al. Vulnerable plaque features on coronary CT angiography as markers of inducible regional myocardial hypoperfusion from severe coronary artery stenoses. Atherosclerosis 2011;219:588-95. [Crossref] [PubMed]
- Schroeder S, Kopp AF, Baumbach A, et al. Noninvasive detection and evaluation of atherosclerotic coronary plaques with multislice computed tomography. J Am Coll Cardiol 2001;37:1430-5. [Crossref] [PubMed]
- Caussin C, Ohanessian A, Ghostine S, et al. Characterization of vulnerable nonstenotic plaque with 16-slice computed tomography compared with intravascular ultrasound. Am J Cardiol 2004;94:99-104. [Crossref] [PubMed]
- Leber AW, Knez A, Becker A, et al. Accuracy of multidetector spiral computed tomography in identifying and differentiating the composition of coronary atherosclerotic plaques: a comparative study with intracoronary ultrasound. J Am Coll Cardiol 2004;43:1241-7. [Crossref] [PubMed]
- Pohle K, Achenbach S, Macneill B, et al. Characterization of non-calcified coronary atherosclerotic plaque by multi-detector row CT: Comparison to IVUS. Atherosclerosis 2007;190:174-80. [Crossref] [PubMed]
- Otsuka K, Fukuda S, Tanaka A, et al. Napkin-ring sign on coronary CT angiography for the prediction of acute coronary syndrome. JACC Cardiovascular Imaging 2013;6:448-57. [Crossref] [PubMed]
- Zhou J, Li Y, Zhang Y, et al. Solitary ground-glass opacity nodules of stage IA pulmonary adenocarcinoma: combination of 18F-FDG PET/CT and high- resolution computed tomography features to predict invasive adenocarcinoma. Oncotarget 2017;8:23312-21. [PubMed]
- Voros S, Rinehart S, Qian Z, et al. Coronary atherosclerosis imaging by coronary CT angiography: current status, correlation with intravascular interrogation and meta-analysis. JACC Cardiovasc Imaging 2011;4:537-48. [Crossref] [PubMed]
- Kashiwagi M, Tanaka A, Kitabata H, et al. Feasibility of noninvasive assessment of thin-cap fibroatheroma by multidetector computed tomography. JACC Cardiovasc Imaging 2009;2:1412-9. [Crossref] [PubMed]
- Ozaki Y, Tanaka A, Tanimoto T, et al. Thin-cap fibroatheroma as high-risk plaque for microvascular obstruction in patients with acute coronary syndrome. Circ Cardiovasc Imaging 2011;4:620-7. [Crossref] [PubMed]
- Li QX, Fu QQ, Shi SW, et al. Relationship between plasma inflammatory markers and plaque fibrous cap thickness determined by intravascular optical coherence tomography. Heart 2010;96:196-201. [Crossref] [PubMed]
- Fujii K, Hao H, Shibuya M, et al. Accuracy of OCT, grayscale IVUS, and their combination for the diagnosis of coronary TCFA: an ex vivo validation study. JACC Cardiovasc Imaging 2015;8:451-60. [Crossref] [PubMed]
- Tomizawa N, Nojo T, Inoh S, et al. Difference of coronary artery disease severity, extent and plaque characteristics between patients with hypertension, diabetes mellitus or dyslipidemia. Int J Cardiovasc Imaging 2015;31:205-12. [Crossref] [PubMed]
- Maurovich-Horvat P, Hoffmann U, Vorpahl M, et al. The napkin-ring sign: CT signature of high-risk coronary plaques? JACC Cardiovasc Imaging 2010;3:440-4. [Crossref] [PubMed]
- Pflederer T, Marwan M, Schepis T, et al. Characterization of culprit lesions in acute coronary syndromes using coronary dual-source CT angiography. Atherosclerosis 2010;211:437-44. [Crossref] [PubMed]
- Nishio M, Ueda Y, Matsuo K, et al. Detection of disrupted plaques by coronary CT: comparison with angioscopy. Heart 2011;97:1397-402. [Crossref] [PubMed]
- Nakazawa G, Tanabe K, Onuma Y, et al. Efficacy of culprit plaque assessment by 64-slice multidetector computed tomography to predict transient no-reflow phenomenon during percutaneous coronary intervention. Am Heart J 2008;155:1150-7. [Crossref] [PubMed]
- Tanaka A, Shimada K, Yoshida K, et al. Non-invasive assessment of plaque rupture by 64-slice multidetector computed tomography--comparison with intravascular ultrasound. Circ J 2008;72:1276-81. [Crossref] [PubMed]
- Donnelly P, Maurovich-Horvat P, Vorpahl M, et al. Multimodality imaging atlas of coronary atherosclerosis. JACC Cardiovasc Imaging 2010;3:876-80. [Crossref] [PubMed]
- Seifarth H, Schlett CL, Nakano M, et al. Histopathological correlates of the napkin-ring sign plaque in coronary CT angiography. Atherosclerosis 2012;224:90-6. [Crossref] [PubMed]
- Maurovich-Horvat P, Schlett CL, Alkadhi H, et al. The napkin-ring sign indicates advanced atherosclerotic lesions in coronary CT angiography. JACC Cardiovasc Imaging 2012;5:1243-52. [Crossref] [PubMed]
- Motoyama S, Sarai M, Narula J, et al. Coronary CT angiography and high-risk plaque morphology. Cardiovasc Interv Ther 2013;28:1-8. [Crossref] [PubMed]
- Maurovich-Horvat P, Ferencik M, Voros S, et al. Comprehensive plaque assessment by coronary CT angiography. Nat Rev Cardiol 2014;11:390-402. [Crossref] [PubMed]
- Schroeder S, Flohr T, Kopp AF, et al. Accuracy of density measurements within plaques located in artificial coronary arteries by X-ray multislice CT: results of a phantom study. J Comput Assist Tomogr 2001;25:900-6. [Crossref] [PubMed]
- Nikolaou K, Becker CR, Muders M, et al. Multidetector-row computed tomography and magnetic resonance imaging of atherosclerotic lesions in human ex vivo coronary arteries. Atherosclerosis 2004;174:243-52. [Crossref] [PubMed]
- Kitagawa T, Yamamoto H, Ohhashi N, et al. Comprehensive evaluation of noncalcified coronary plaque characteristics detected using 64-slice computed tomography in patients with proven or suspected coronary artery disease. Am Heart J 2007;154:1191-8. [Crossref] [PubMed]
- Cademartiri F, Mollet NR, Runza G, et al. Influence of intracoronary attenuation on coronary plaque measurements using multislice computed tomography: observations in an ex vivo model of coronary computed tomography angiography. Eur Radiol 2005;15:1426-31. [Crossref] [PubMed]


