Cetuximab in the treatment of advanced non-small cell lung cancer: FISHing for a miraculous catch
Non-small cell lung cancer (NSCLC) is divided in two main histologies: non-squamous and squamous histotypes. In metastatic non-squamous NSCLC patients, the oncogene-addicted subgroups are characterized by the presence of epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK) and proto-oncogene tyrosine-protein kinase 1 (ROS1) deregulations which can benefit, in the current practice, by specific tyrosine kinase inhibitors (TKIs). The programmed death-ligand 1 (PD-L1) ≥50% selects another subgroup which can benefit, in the clinical practice, by first-line pembrolizumab. In squamous NSCLC patients, the only biomarker able to select a group of patients who can benefit from a personalized therapy with pembrolizumab is that with strong positive PD-L1. Overall, about 60% of non-squamous NSCLC patients and approximately 75% of squamous ones still receive chemotherapy as first-line approach (1,2).
However, even in absence of mutations, EGFR overexpression on the surface of tumor cells can regulate cell proliferation and survival, representing the rationale to exploit alternative ways to inhibit EGFR axis through monoclonal antibodies (mAbs) such as cetuximab and necitumumab (3). The EGFR overexpression detected by immunohistochemistry (IHC) was found in approximately 80–90% of NSCLC patients regardless of histotypes (4). Based on this finding, several trials investigated anti-EGFR mAbs in combination with chemotherapy. An individual patient data meta-analysis included 2018 advanced NSCLC patients from four randomized phase II/III trials investigating the addition of cetuximab to first-line platinum-based chemotherapy. Median overall survival (OS) was 10.3 months for the cetuximab plus chemotherapy arm vs. 9.4 months for the chemotherapy alone [hazard ratio (HR), 0.88; P=0.009), the median progression-free survival (PFS) was 4.7 vs. 4.5 months (HR, 0.90; P=0.045) and the objective response rate (ORR) was 32.2% vs. 24.4% [odds ratio (OR), 1.46; P<0.001], respectively. The safety profile of the addition of cetuximab to chemotherapy resulted manageable. Overall, this meta-analysis indicated a favorable benefit/risk ratio for the addition of cetuximab to platinum-based, first-line chemotherapy for advanced NSCLC patients (5). However, regulatory authorities considered these data insufficient for recommending the approval, judging the OS benefit and risks as disproportional. Hence, the results observed clearly indicated that there was a consistent fraction of NSCLC patients deriving little or no benefit from cetuximab therapy, confirming the importance of a proper patient selection. In this context, EGFR overexpression might be crucial for cetuximab activity. In the largest FLEX trial, which enrolled only EGFR overexpressing NSCLC patients (6), the outcomes were evaluated according to EGFR expression and using a specific histoscore (H-score), demonstrating that the addition of cetuximab to chemotherapy produced a significant benefit only in individuals with an EGFR score equal or greater than 200 (7). Nevertheless, H-score as well other IHC scoring systems resulted as weak predictors for cetuximab benefit, thus highlighting the need of a more reliable biomarker (8). However, interestingly, in the FLEX trial, the greatest OS improvement was obtained in patients with squamous cell histology (HR, 0.80), with an increased risk of adverse events, particularly febrile neutropenia (6).
Necitumumab was also investigated in phase III trials either in combination with pemetrexed and cisplatin without increasing OS of previously untreated metastatic non-squamous NSCLC patients (9) and in combination with gemcitabine and cisplatin, improving OS in patients with advanced squamous NSCLC patients (10).
Bevacizumab, a monoclonal antibody directed against vascular endothelial growth factor (VEGF), in combination with paclitaxel and carboplatin, is one of the standard-of-care for first-line therapy of non-oncogene addicted non-squamous NSCLC patients (2).
EGFR gene copy number assessed by fluorescent in situ hybridization (FISH) and defined according to the Colorado Criteria, has proven, retrospectively, to be potentially useful for selection of NSCLC patients for cetuximab and necitumumab plus chemotherapy (11-14).
These considerations became the background for designing the South Western Oncology Group (SWOG) S0819 trial. Herbst et al. investigated the safety and effectiveness of first-line therapy with carboplatin plus paclitaxel with or without bevacizumab either with cetuximab or without in patients with advanced NSCLC. The trial was designed to validate the EGFR FISH assay as predictive biomarker for cetuximab benefit. The co-primary endpoints were OS in the whole study population and PFS in patients with EGFR FISH-positive, using the Colorado Criteria (15). A total of 1,313 patients were randomly assigned to the control group (n=657; 277 with chemotherapy plus bevacizumab and 380 with chemotherapy only) or to the cetuximab group (n=656; 283 with chemotherapy plus bevacizumab and 373 with chemotherapy only). Patients were allocated in the no-bevacizumab arms if they were unsuitable for bevacizumab due to medical reasons or physician opinion. EGFR FISH-positivity was detected in 400 patients (41%) out of the 976 assessable. In the total population, median PFS was 4.5 months in the control arm and 4.6 in the cetuximab group [HR, 0.99; 95% confidence interval (CI), 0.88–1.10; P=0.83], median OS was 9.2 and 10.9 months (HR, 0.93; 95% CI, 0.83–1.04; P=0.22) and ORR 36% vs. 42% (P=0.060), respectively. In the EGFR FISH-positive subpopulation, the median PFS was 4.8 vs. 5.4 months (HR, 0.92; 95% CI, 0.75–1.12; P=0.40) between the control group and the cetuximab group, respectively. Median OS was 9.8 vs. 13.4 months (HR, 0.81; 95% CI, 0.66–1.00; P=0.054) and ORR was 43% vs. 47% (P=0.48), respectively. Severe adverse events, mainly hematological toxicities, fatigue and skin rash occurred in 9% of patients in the cetuximab group and 5% of patients in the control group. Toxic deaths occurred in 6% of patients in the cetuximab group and 2% of patients in the control group. Bevacizumab was not associated with OS or PFS in the entire population or according to EGFR FISH status, with a moderate increase of toxicity (15).
However, the most compelling finding of the study emerged from a pre-specified subgroup analysis, looking at the potential impact of tumor histology on cetuximab outcome. In the EGFR FISH-positive subpopulation with squamous cell histology, median OS was 11.8 months in the cetuximab group and 6.1 months in the control group (HR, 0.58; 95% CI, 0.36–0.86; P=0.0071), although no difference in PFS was reported between these two treatment subgroups (median 4.5 vs. 2.8 months, respectively. HR, 0.68; 95% CI, 0.46–1.01; P=0.055). In patients with EGFR FISH-negative with squamous cell histology or patients with non-squamous histology regardless of EGFR FISH status, OS and PFS did not differ among control and cetuximab arms (15).
To date, the SWOG S0819 is the largest trial having a biomarker-driven co-primary end point. Furthermore, even if the study should be considered as a negative trial, it supports the conviction that tumor histology is more than a sum of morphological features, thus reflecting different tumor biology. Squamous cell carcinoma rarely harbors EGFR mutations while the EGFR overexpression is frequent, underlying both an EGFR dependence and a potential sensitivity to EGFR-inhibition (6,10,16). In the SQUIRE trial, addressed exclusively to squamous NSCLC, the addition of necitumumab to front-line cisplatin-gemcitabine translated into an improved OS with a median of 11.5 vs. 9.9 months (HR, 0.84; P=0.01) (10). A subgroup analysis showed a trend toward better PFS and OS for EGFR FISH-positive patients (17) (Table 1). Thus, SWOG S0819 and SQUIRE findings seem to apparently answer an important question: the optimal front-line therapy for advanced squamous NSCLC. However, in the clinical practice, we should perform the EGFR FISH assay in the presence of squamous histology in order to administer an anti-EGFR mAb in case of positivity. In this way, should we consider the EGFR FISH-positive squamous cell carcinoma an addicted population? This is not correct, in fact, although it is a common opinion to consider a cancer population carrying a specific molecular event as target-driven, EGFR FISH-positive squamous cell lung carcinoma looks very different from the other addicted NSCLCs, mainly because so far, no anti-EGFR mAbs demonstrated activity when used alone in this subgroup. In addition, it is not possible to exclude that anti-EGFR benefit should be restricted to the EGFR amplified group only, thus reducing from approximately 40% to 7%—the amount of patients potentially benefiting from anti-EGFR mAb, as suggested by an analysis of granular EGFR FISH data from the SQUIRE trial (14). Finally, FISH assay has some limitations, including reproducibility, the need of tissue specimens and costs.
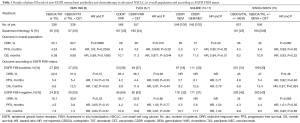
Full table
Considering that most of patients are still receiving first-line chemotherapy regardless of histology, the unmet need is to select other subgroups of patients who can benefit from a personalized treatment. The potential of EGFR FISH selection in squamous NSCLC is further complicated by the introduction of immunotherapy, which is radically changing the treatment landscape of NSCLC. In fact, on 20th March 2018, a press release announced that the phase III IMpower131 trial met its co-primary end point of PFS, still waiting for the final OS results. The trial demonstrated that the combination of atezolizumab, an anti-PD-L1 mAb, to carboplatin plus nab-paclitaxel reduced the risk of PFS and death compared to chemotherapy alone as first-line treatment of advanced squamous NSCLC patients, regardless of PD-L1 expression (18).
In conclusion, SWOG S0819 results should be not sufficient to find a place for cetuximab in the treatment algorithm of advanced NSCLC, even in squamous histology and in EGFR FISH-positive patients. Additional studies specifically focused on EGFR FISH amplified population are strongly encouraged to definitively address the role of anti-EGFR mAb in such setting and possibly explore the potential of combining anti-EGFR mAbs strategies with immunotherapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: Antonio Rossi is Speaker Bureau for Eli Lilly, MSD, Boehringer Ingelheim, AstraZeneca, Roche. The other author has no conflicts of interest to declare.
References
- Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v1-27. [Crossref] [PubMed]
- Hanna N, Johnson D, Temin S, et al. Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:3484-515. [Crossref] [PubMed]
- Pirker R. Epidermal growth factor receptor-directed monoclonal antibodies in nonsmall cell lung cancer: an update. Curr Opin Oncol 2015;27:87-93. [Crossref] [PubMed]
- Rusch V, Baselga J, Cordon-Cardo C, et al. Differential expression of the epidermal growth factor receptor and its ligands in primary non-small cell lung cancers and adjacent benign lung. Cancer Res 1993;53:2379-85. [PubMed]
- Pujol JL, Pirker R, Lynch TJ, et al. Meta-analysis of individual patient data from randomized trials of chemotherapy plus cetuximab as first-line treatment for advanced non-small cell lung cancer. Lung Cancer 2014;83:211-8. [Crossref] [PubMed]
- Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 2009;373:1525-31. [Crossref] [PubMed]
- Pirker R, Pereira JR, von Pawel J, et al. EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the phase 3 FLEX study. Lancet Oncol 2012;13:33-42. [Crossref] [PubMed]
- Khambata-Ford S, Harbison CT, Hart LL, et al. Analysis of potential predictive markers of cetuximab benefit in BMS099, a phase III study of cetuximab and first-line taxane/carboplatin in advanced non-small-cell lung cancer. J Clin Oncol 2010;28:918-27. [Crossref] [PubMed]
- Paz-Ares L, Mezger J, Ciuleanu TE, et al. Necitumumab plus pemetrexed and cisplatin as first-line therapy in patients with stage IV non-squamous non-small-cell lung cancer (INSPIRE): an open-label, randomised, controlled phase 3 study. Lancet Oncol 2015;16:328-37. [Crossref] [PubMed]
- Thatcher N, Hirsch FR, Luft AV, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol 2015;16:763-74. [Crossref] [PubMed]
- Hirsch FR, Herbst RS, Olsen C, et al. Increased EGFR gene copy number detected by fluorescent in situ hybridization predicts outcome in non-small-cell lung cancer patients treated with cetuximab and chemotherapy. J Clin Oncol 2008;26:3351-7. [Crossref] [PubMed]
- Herbst RS, Kelly K, Chansky K, et al. Phase II selection design trial of concurrent chemotherapy and cetuximab versus chemotherapy followed by cetuximab in advanced-stage non-small-cell lung cancer: Southwest Oncology Group study S0342. J Clin Oncol 2010;28:4747-54. [Crossref] [PubMed]
- Kim ES, Moon J, Herbst RS, et al. Phase II trial of carboplatin, paclitaxel, cetuximab, and bevacizumab followed by cetuximab and bevacizumab in advanced nonsquamous non-small-cell lung cancer: SWOG S0536. J Thorac Oncol 2013;8:1519-28. [Crossref] [PubMed]
- Genova C, Socinski MA, Hozak RR, et al. EGFR gene copy number by FISH may predict outcome of necitumumab in squamous lung carcinomas: analysis from the SQUIRE study. J Thorac Oncol 2018;13:228-36. [Crossref] [PubMed]
- Herbst RS, Redman MW, Kim ES, et al. Cetuximab plus carboplatin and paclitaxel with or without bevacizumab versus carboplatin and paclitaxel with or without bevacizumab in advanced NSCLC (SWOG S0819): a randomised, phase 3 study. Lancet Oncol 2018;19:101-14. [Crossref] [PubMed]
- Lopez-Malpartida AV, Ludena MD, Varela G, et al. Differential ErbB receptor expression and intracellular signaling activity in lung adenocarcinomas and squamous cell carcinomas. Lung Cancer 2009;65:25-33. [Crossref] [PubMed]
- Paz-Ares L, Socinski MA, Shahidi J, et al. Correlation of EGFR-expression with safety and efficacy outcomes in SQUIRE: a randomized, multicenter, open-label, phase III study of gemcitabine-cisplatin plus necitumumab versus gemcitabine-cisplatin alone in the first-line treatment of patients with stage IV squamous non-small-cell lung cancer. Ann Oncol 2016;27:1573-9. [Crossref] [PubMed]
- Phase III IMpower131 study showed Roche’s TECENTRIQ (atezolizumab) plus chemotherapy (carboplatin and Abraxane) reduced the risk of disease worsening or death in the initial treatment of people with a type of advanced squamous lung cancer. Available online: https://www.roche.com/media/store/releases/med-cor-2018-03-20.htm. Accessed on March 21, 2018.


