Mucinous cystadenocarcinoma arising from mucinous cystadenoma of the lung: case report and review of the literature
Introduction
Mucinous cystadenoma is defined as “a localized cystic mass filled with mucin and surrounded by a fibrous wall lined by well-differentiated columnar mucinous epithelium” (1). The benign tumor is commonly found in pancreas and ovaries, as well as in the appendix (2-4). In the lung, however, only a few cases have been reported world-wide (5-7). The usually unilocular tumor can be located in the periphery of any lobe of the lung. Since the first description by Eck et al. in 1969 and a report by Sambrook Gowar in 1978, primary pulmonary mucinous cystic neoplasia has been recognized as a separate entity (8-10). Following the classification of Gao et al. according to pathologic features there is benign pulmonary mucinous cystadenoma (PMCA), pulmonary mucinous tumor of borderline malignancy [with atypia; postmortem computed tomography angiography (PMCT-A)] and pulmonary mucinous cystadenocarcinoma (PMCAC) (8).
The main differential diagnoses for PMCA and PMCAC are bronchioalveolar carcinoma, solitary metastatic mucinous tumors from other organs and bronchial mucous gland adenoma (11). According to the 2015 WHO classification of tumors of the lung, pleura, thymus and heart, the term mucinous cystadenocarcinoma has been discontinued and the entity has been included under the category of colloid adenocarcinoma (12). For simplification, especially with regard to the review we have kept the old term “mucinous cystadenocarcinoma” throughout the manuscript.
Case presentation
A 58-year old female with a history of recurrent airway infections was admitted to the hospital with cough, dyspnea and pain in the left lower side of the chest. Serum C-reactive protein (CRP) levels were only moderately elevated. The chest X-ray revealed a large opacity in the left upper lobe of the lung. A CT scan was initiated, confirming a 12.0 cm × 7.5 cm smooth margined homogeneous mass in the left upper lobe with adjacent atelectasis, inflammatory infiltrate and mediastinal lymphadenopathy (Figure 1).

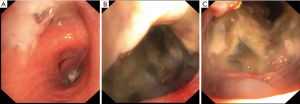
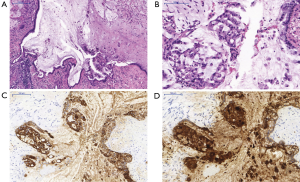
Bronchoscopy revealed distortion and partial atelectasis of the segments 1–3 of the left upper lobe and initial occlusion of segment 4 (Figure 2A). When probed, a lot of mucous was drained, revealing a rather large necrotic cavern (Figure 2B,C). Ample biopsies were taken and endobronchial ultrasound-guided transbronchial fine needle aspiration biopsy (EBUS-TBNA) was performed to evaluate the mediastinal lymph nodes. Malignancy could not be excluded by histopathological findings alone, so the patient underwent surgical therapy. Anterolateral thoracotomy was performed with resection of the left upper lobe and systematic mediastinal lymphadenectomy. Macroscopic examination showed a well-circumscribed cystic mass filled with mucus and a maximum diameter of 12 cm. Histologically the cyst was lined by tall mucinous epithelium, while nuclear atypia was mild and polarity was still conserved. Thus, the tumor was initially interpreted as mucinous cystadenoma (Figure 3A) Immunohistochemical analysis of the mucin producing epithelial cells showed expression of CEA and CK7. In pneumocytes and respiratory epithelium CK7 staining was also positive. However, further analyses revealed focal singlet cells within the mucus, which were diagnostic of cystadenocarcinoma arising from the cystadenoma (Figure 3B,C,D). All lymph nodes were found to be cancer free. There weren’t any postoperative complications. Antibiotic therapy was started with cefuroxime right after surgery and later changed to moxifloxacin following the identification of the pathogen as Klebsiella pneumoniae. There was a gradual decrease in serum inflammatory parameters.
About 1 year after initial surgery the patient is doing well and is undergoing regular follow-ups including CT-scans and bronchoscopy.
Discussion and review of the current literature
We conducted a systemic search for “PMCA”, “pulmonary mucinous cystadenoma”, “PMCAC”, “pulmonary mucinous cystadenocarcinoma” and “PMCT-A” in Medline and the Cochrane Library. To our knowledge, 14 cases of PMCA have been reported in the English literature so far with one being describes as unusual mucinous cyst (5-8,10,13-16). Patients’ age ranges from 32–75 years (median 61 years) and the tumor seems to be more common in females (10 females vs. 5 males). They were located in the periphery of any lobe of the lung, with a preference towards the right side (11 vs. 4 cases). The median size was 5 cm (range, 0.8–15 cm), which makes our case one of the largest tumors diagnosed so far. In some cases, the cystadenoma could be reached via bronchoscopy, yet a definite diagnosis could never be established by endoscopic biopsy alone (Table 1). Preoperative diagnosis is challenging because more than 90% of the tumor bulk consist of mucin (5), leaving only a small number of cells. Radiographically PMCA appears as a well-defined, homogeneous lesion. In one case of pediculate PMCA, the tumor proved to be difficult to distinguish from a pleural tumor due to its location close to the chest wall in the right upper lobe (16). Inflammation and adjacent atelectasis only seem to be present after a certain enlargement of the lesion due to compression and distortion of the surrounding tissue. Patients then develop recurrent pneumonia, cough and chest pain. Except for one patient presenting with hemoptysis originating from a 5-cm PMCA, the other lesions were detected incidentally. Aside from other malignant and benign tumors originating from the lungs, solitary metastatic mucinous tumors from other organs, especially from the ovary and pancreas, are important differential diagnoses. Thorough clinical and radiologic evaluation is, as always, mandatory (17).

Full table
Thoracotomy was performed for tumor resection for all but two cases (video-assisted thoracoscopy), followed by lobectomy in eight cases and wedge resection in seven cases. A benign lesion confirmed in intraoperative frozen section led to the surgery being finished after wedge resection. In our case enlargement of the mediastinal lymph nodes was present in addition to a large tumor of unclear dignity, therefore complete oncological resection was performed.
Immunohistochemical studies usually show positivity for pan-cytokeratin (CK) and in some cases CEA (5), in accordance with the findings from our case. Stains for thyroid transcription factor-1 (TTF-1) are usually negative. In our case initial interpretation was difficult due to the fact that most lining epithelial cells showed only mild atypia, which is in agreement with PCMA. Upon thorough immunohistochemical workup, especially due to CEA positivity, intramucinous singlet cells were diagnostic for invasive carcinoma, in this case a mucinous cystadenocarcinoma arising from a PCMA. Rigorous and precise sampling and microscopic evaluation including the intracystic mucinous masses are therefore pivotal for the assessment of malignancy in PCMA.
KRAS point mutations have been suggested to be associated with tumor development in mucinous neoplasms originating from the ovary, appendix or pancreas (4,5). In the literature, there has been only one case of mucinous cystadenoma in the lung that was analyzed for KRAS and EGRF mutations and both were negative.
We prepared micro dissection of the tumor and extracted DNA from the cancer cells, which were then analyzed for 15 different well-known driver mutations using next-generation sequencing (TruSight Tumor 15, Illumina, USA). We found a driver mutation typical for carcinomas in exon 12 of the KRAS gene (p.G12V). In concordance with this finding, EGFR exons 18–21, as well as BRAF exons 11 and 15 revealed wild type sequences. Furthermore, two more polymorphisms that have been documented in the dbSNP (database of Single Nucleotide Polymorphism; PIK3CA exon 9 p.R524K; TP53 exon 4 p.P72R) were identified. On the basis of our molecular pathological investigations with detection of a typical KRAS mutation the transformation into a mucinous cystadenocarcinoma could be further verified.
Conclusions
PMCA is a rare benign tumor that should be kept in mind as a differential diagnosis when investigating patients with solitary, radiographically well-defined, mucinous lesions in the lung. Most patients are not experiencing any symptoms. There are, however, cases like ours where the lesion has grown to cause distortion in the surrounding tissue and inflammation and atelectasis are present.
Although the prognosis for patients with PMCA is usually good, this is the third case reporting a focal adenocarcinoma arising from a mucinous cystadenoma (13). Moreover, there is another case reporting focal atypia in PMCA consistent with adenocarcinoma (14) and one case reporting recurrence of PMCA 20 years post initial surgery despite initial complete (R0) resection (6).
Due to a very limited number of cases reported so far, the biology of this tumor and the underlying pathogenesis of malignant transformation remains unclear but our molecular analyses reveal that driving KRAS mutations, which are typical for mucinous carcinomas of the lung, may as well be one mechanism for the development of mucinous cystadenocarcinoma arising from PCMA. To further corroborate those findings and to determine if complete oncologic resection might be beneficial, more studies are needed.
Acknowledgements
None
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Travis WD, Brambilla E, Muller-Hermelink HK, et al. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. IARC Press: Lyon, 2004.
- McFarlane ME, Plummer JM, Bonadie K. Mucinous cystadenoma of the appendix presenting with an elevated carcinoembryonic antigen (CEA): Report of two cases and review of the literature. Int J Surg Case Rep 2013;4:886-8. [Crossref] [PubMed]
- Ali S, Bashir A. Giant mucinous cystadenoma: case report with review of literature. Gland Surg 2014;3:207-10. [PubMed]
- Brown J, Frumovitz M. Mucinous tumors of the ovary: current thoughts on diagnosis and management. Curr Oncol Rep 2014;16:389. [Crossref] [PubMed]
- Haruki T, Nakamura H, Taniguchi Y, et al. Pulmonary mucinous cystadenoma: a rare benign tumor of the lung. Gen Thorac Cardiovasc Surg 2010;58:287-90. [Crossref] [PubMed]
- Matsuo T, Yusuke Kimura N, Takamori S, et al. Recurrent pulmonary mucinous cystadenoma. Eur J Cardiothorac Surg 2005;28:176-7. [Crossref] [PubMed]
- Roux FJ, Lantuéjoul S, Brambilla E, et al. Mucinous cystadenoma of the lung. Cancer 1995;76:1540-4. [Crossref] [PubMed]
- Gao ZH, Urbanski SJ. The spectrum of pulmonary mucinous cystic neoplasia: a clinicopathologic and immunohistochemical study of ten cases and review of literature. Am J Clin Pathol 2005;124:62-70. [Crossref] [PubMed]
- Eck H, Haupt R, Rothe G. Die gut- und bosartigen Lungengeschwulste. Henke F, Lubarsch O, eds Handbuch der speziellen pathologischen Anatomic and Histologie Berlin, Springer Verlag, 1969;11:59-61.
- Sambrook Gowar FJ. An unusual mucous cyst of the lung. Thorax 1978;33:796-9. [Crossref] [PubMed]
- Couraud S, Isaac S, Guibert B, et al. Bronchial mucous gland adenoma revealed following acute pneumonia. Interact Cardiovasc Thorac Surg 2012;14:347-9. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Davison AM, Lowe JW, Costa PD. Adenocarcinoma arising in a mucinous cystadenoma of the lung. Thorax 1992;47:129-30. [Crossref] [PubMed]
- Dixon AY, Moran JF, Wesselius LJ, et al. Pulmonary mucinous cystic tumor. Case report with review of the literature. Am J Surg Pathol 1993;17:722-8. [Crossref] [PubMed]
- Guimaraes AR, Wain JC, Mark EJ, et al. Mucinous cystadenoma of the lung. AJR Am J Roentgenol 2004;183:282. [Crossref] [PubMed]
- Igai H, Okumura N, Ohata K, et al. Pediculate mucinous cystadenoma difficult to differentiate from pleural tumor. Ann Thorac Surg 2008;85:1807-9. [Crossref] [PubMed]
- Ishibashi H, Moriya T, Matsuda Y, et al. Pulmonary mucinous cystadenocarcinoma: report of a case and review of the literature. Ann Thorac Surg 2003;76:1738-40. [Crossref] [PubMed]


