Effects of para-toluenesulfonamide intratumoral injection on pulmonary adenoid cystic carcinoma complicating with severe central airway obstruction: a 5-year follow-up study
Introduction
Pulmonary adenoid cystic carcinoma (ACC), a rare type of non-small cell lung carcinoma (NSCLC), may develop severe malignant airway obstruction (SMAO) which is frequently life-threatening (1). Overall, the 5-year survival of patients with ACC is ~52% (2). However, surgical resection is limited by the size and location of ACC. The 5-year survival of patients with inoperable ACC is merely 33%. To date, there is no effective therapeutic option for ACC-SMAO, highlighting urgent unmet needs of developing novel therapeutic regimens.
Para-toluenesulfonamide (PTS) is a low-molecular-weight organic compound that confers powerful anti-tumor effects in mouse models by eliciting tumor necrosis whilst resulting in minimal injury to adjacent normal tissues (3). In a multicenter, single-arm, open-label trial (www.chictr.org/cn, No.: ChiCTR-TNC-12002648), PTS intratumoral injection significantly alleviated airway obstruction and improved lung function in patients with NSCLC-SMAO (4). However, therapeutic responses of ACC-SMAO to PTS injection might differ from that of other pathological subtypes of NSCLC. Because the observation spanned for only 1 year, the long-term survival following PTS treatment remains unclear. We hypothesized that PTS intratumoral injection would be equally, if not more, effective for ACC-SMAO. Here, we specifically analyzed the effects of PTS intratumoral injection in patients with ACC-SMAO by extending follow-up to 5 years (5).
Methods
This is a sub-analysis of the patients with ACC who were included in the analysis of our previous paper. Therefore, this study has extended from the previous study by specifically analyzing the survival of patients with surgically inoperable ACC-SMAO (5).
Between October 2009 and January 2011, we prospectively recruited eight hospitalized patients with ACC-SMAO from four medical centers in China. Eligibility criteria included: (I) 18–83 years of age; (II) physician-diagnosed ACC according to tumor biopsy findings and ACC cell morphological classification as described previously (6). SMAO was defined as the ratio of tumor/trachea diameter being 0.50 or greater, the ratio of tumor/main bronchi diameter being 0.67 or greater, or the longest tumor diameter being greater than 0.5 cm (evaluated with bronchoscopy); (III) lesions suitable for bronchoscopic treatment; (IV) tumor sizes measurable with computed tomography (CT); (V) no thrombocytopenia. Key exclusion criteria were cerebral metastasis and severe cardiovascular diseases. Protocol approval was obtained from Ethics Committee of participating sites and China Food and Drug Administration [No.: 2009L03443; Medical Ethics Year 2009 (the 12th) and Medical Ethics Year 2016 (the 33rd) for follow-up study]. Informed consent forms were signed before enrollment.
This was a phase III, multicenter, non-randomized, single-arm, open-label trial. Randomized, double-blind, parallel-group study was not conducted because it was deemed unethical, according to local ethics committee and China Food and Drug Administration. Both physicians and patients were aware of treatment allocation. Pre-clinical toxicity studies have established the initial dose in human to be 3.0 mL/d. A phase I dose-escalation study in human has confirmed that the daily tolerable dose for intratumoral injection of PTS was 10 mL, which was selected as the daily dose in the present study. Each ampoule contained 5 mL of PTS, and up to two ampoules of PTS would be administered to the patients per each treatment day (diluted in ethanol absolute).
The methodology of PTS intratumoral injection has been described previously (5). Before injection, 5 mL PTS (Lot No.: 070109-070111; Guangdong Dari Chemicals Inc., Guangzhou, China) was diluted with 2 mL ethanol anhydride into a 10 mL sterile syringe (ethanol concentration: 28.5%). PTS-ethanol mixture was intratumorally injected to tumor’s root. Within the first year, patients with ACC-SMAO received PTS injection for 2−3 sessions weekly, with 2 weeks as a single course. Four doses were mandatory for the initial course, but the doses could be adjusted for the remaining courses. After the 4 doses of PTS administration, no further PTS dosing would be performed when the airway tumor size had been reduced by at least 50%.
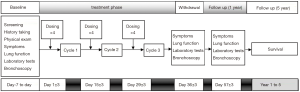
Pre- and post-treatment assessments, including vital sign assessment, dyspnea index, chest CT, were performed shortly before PTS injection and at day 30 after treatment. We also extended the observation period to five years to determine overall survival (OS) (Figure 1). Adverse events were, if any, documented.
The primary endpoint was the airway objective response rate (ORR), assessed with chest CT at day 30, based on Response Evaluation Criteria In Solid Tumor (RECIST). The CT assessment was used to evaluate the airway obstruction rate and the magnitude of alleviation of airway obstruction at day 30 post-treatment. Meanwhile, bronchoscopic assessment of airway tumor area and airway obstruction at day 30 post-treatment was also conducted. K-PACS (IMAGE Information Systems Ltd. London, United Kingdom) and MensurePlus (Guangzhou Institute of Respiratory Health, Guangzhou, China) was used for CT and bronchoscopic image measurement, respectively. Bronchoscopic results, which demonstrated similar trends of changes with chest CT, are listed in Table S1. The key secondary endpoint was the OS at 5 years post-treatment. Other secondary endpoints included: (I) Transitional Dyspnea Scale (TDI), measured at day 30 and 1-year post-treatment; (II) forced expiratory volume in one second (FEV1), measured at day 30 post-treatment; and (III) the ratio of FEV1 to forced vital capacity (FEV1/FVC), measured at day 30 post-treatment. Adverse events (AE) and severe adverse events (SAE) were recorded and presented as numbers and percentages.
Full table
We analyzed the endpoints based on the modified intention-to-treat principle. Full-analysis set included patients who received at least one dose of PTS (n=8). All patients had received at least 4 injections in this study. All analyses were performed for the full-analysis set. Data were processed with Graphpad Prism 5.0 (Graphpad Inc., USA). Full-analysis set included eight patients who had received PTS treatment. Numeric data were described as mean ± standard deviation, whereas treatment effect was presented as mean difference [95% confidence interval (95% CI)]. Survival was analyzed with log-rank test.
Results
Baseline characteristics
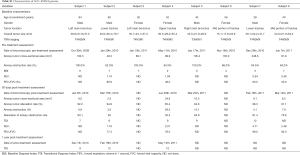
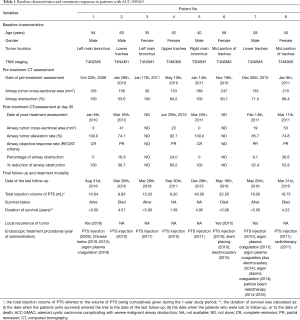
The cohort consisted of mostly middle-aged patients who had predominantly stage IIIB and IV ACC-SMAO located in the trachea (n=5) or main bronchus (n=3). All patients were assessed for injection and have received at least 4 injections. At baseline, mean airway tumor cross-sectional area was 153.3 mm2 (n=8), and the mean airway obstruction rate was 86.1% (n=8) (Table 1). Two patients without CT assessment at day 30 post-treatment were not included in efficacy analysis. At the investigators’ discretion, two patients (No. 7 and 8) had pre-treatment stent insertion for alleviating airway obstruction. The stents were removed after PTS treatment. No other concomitant treatment for relieving airway obstruction was performed for other patients.
Full table
Primary endpoints
Two patients without CT assessment at 30 days post-treatment were excluded from the efficacy analysis. According to the RECIST criteria, the airway ORR reached 100% [33.3% complete remission (CR) and 66.7% partial remission (PR)]. PTS treatment (mean: 17.8 mL; range, 8.2–40.0 mL) reduced the airway tumor size from 158.2 to 22.7 mm2 and the average airway obstruction rate decreased from 83.1% to 14.4% (n=6).
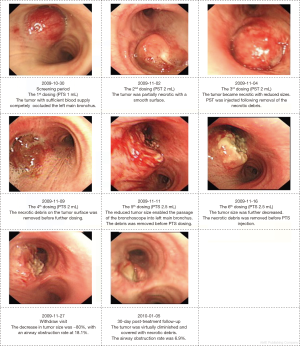
Corresponding results were obtained by bronchoscopy to further demonstrate the anti-tumor effects of PTS (Table S1). At day 30, PTS treatment significantly reduced the mean airway tumor area from 116.2 to 13.09 mm2, and decreased the mean airway obstruction rate from 85.8% to 10.8% (n=6). The typical changes in tumor sizes during endoscopic examination are shown in Figure 2.
Secondary endpoints
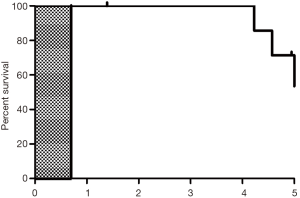
No patient succumbed at one year after PTS treatment, whereas at 5 years two patients (25.0%) succumbed, four patients (50.0%) were still alive, and 2 patients (25.0%) were lost to follow-up (follow-up duration: 1.39 and 4.95 years, respectively). Therefore, the 5-year overall survival rate was 50.0%. The median survival duration was 4.98 years (range, 1.39–5.00 years) (Figure S1). Four patients (50.0%) were deemed to have stable disease.
Compared with BDI score, the TDI score increased significantly at 30 days after treatment (pre-treatment: 3.60±2.88; post-treatment: 9.00±1.87; mean difference: 5.40; 95% CI, 0.31–10.49; P<0.05). There was a progressive increase among the three patients (subjects No. 1, 2, and 4) who attended the 1 year follow-up assessment.
At follow-up, four patients had undergone spirometry, therefore spirometric data were only available in four patients in total. There was a non-significant increase in FEV1 (from 1.18±0.71 liters to 2.60±1.51 liters, mean difference: 1.42 liters; 95% CI, −1.87 to 4.71 liters; P=0.264) and FEV1/FVC (from 54.7±21.4 to 84.1±10.2, mean difference: 29.3; 95% CI, −8.5 to 67.1; P=0.09) in three out of four patients, although the trend was not statistically significant due to the limited number of patients included for analysis.
Adverse events
Adverse events (AEs) were reported in 75% (n=6) of patients, of whom 33.3% (n=2) and 66.7% (n=4) were rated as mild and moderate, respectively. No SAE was reported. The only one possible drug-related AE reported was tachypnea (12.5%, n=1), which was a reversible, mild and respiratory system related Grade 1 AE according to CTCAE (Common Terminology Criteria for Adverse Events) definition.
The most common AEs were injection-site hemorrhage (25%, n=2) and fever (25%, n=2) according to the Preferred Terms classification. Both AEs belonged to the general disorders and injection-site events (50%, n=4) according to the Systemic Organ Classification. Other AEs included cough (25%, n=2), tachypnea (12.5%, n=1), hemoptysis (12.5%, n=1), loss of appetite (12.5%, n=1), and abdominal pain and vomiting (12.5%, n=1). See Table S2 for further details.

Full table
No significant abnormalities (including bone marrow inhibition) in laboratory tests were noted (data not shown).
Discussion
Our study demonstrated that PTS rapidly debulked ACC-SMAO, resulting in a considerable improvement of 5-year survival rate.
Aside from the longer survival in patients with ACC-SMAO, we also compared the response rate of ACC-SMAO and other patients with non-small cell lung cancer in our previously published study (5). The airway ORR of patients with ACC-SMAO and other patients with lung cancer was 75.0% and 50.8% (chi-square: 1.66, P=0.20), respectively. Despite a significant difference which might be due to our small sample sizes, there was a trend towards greater airway ORR which is reflective of the therapeutic responses between ACC-SMAO and other pathological subtypes of non-small cell lung cancer.
ACC differs from other pathological subtypes of lung cancer because the diagnosis of ACC could be frequently delayed due to the normal or near-normal manifestations on chest radiography or CT (7). However, upon diagnosis, the development of severe dyspnea due to large tumor sizes could have rendered the patients unsuitable for surgical removal. Further complicating the clinical management of ACC is the limited response to chemotherapy and radiotherapy (8). Moreover, ACC-SMAO remains challenging for respiratory physicians due to rapid recurrence that frequently warrants bronchoscopic interventions (9). Although silicone stent placement effectively ameliorated airway obstruction, they cannot be removed from airways (10). An advantage of PTS administration might be the minimal need for foreign body placement. Moreover, despite that patients with ACC reportedly had good long-term outcomes (e.g., up to 10 years) (9,11), few or none of them had developed SMAO on presentation. Our findings were unique in that we have demonstrated the efficacy of PTS injection in the most severe form of pulmonary ACC. The patients enrolled in our study had notably longer survival than those of the general patients with stage IV NSCLC, possibly because of their slow growth of ACC-SMAO. The timely alleviation of major airway obstruction with PTS could be clinically appealing when virtually no other options were available. Because of the safety and effectiveness for alleviating large-airway obstruction, PTS intratumoral injection might be a novel, effective therapy for inoperable ACC-SMAO, particularly in developing countries where sophisticated medical facilities (e.g., electrocautery) are lacking.
Nevertheless, our study was limited by the finite post-treatment assessments of tumor sizes and dyspnea index that were conducted at day 30 only. We cannot address how PTS injection led to improved outcomes using other clinically relevant parameters (e.g., lung function). Therefore, larger trials which incorporate more comprehensive assessments to confirm the efficacy and safety of PTS in patients with ACC-SMAO are warranted.
Acknowledgements
We truly appreciate the dedication of Professor Q Li (Shanghai General Hospital, Shanghai, China) for recruiting some of the patients. We highly appreciate the insightful suggestions from Drs. W Huang, E Tu, M Hsiao, P Hsiao, M Lin, J Chang, and B Wang (Gongwin BioPharm Co., Ltd., Taiwan; PTS International Inc., USA). We also thank Guangdong Dari Chemicals Inc. (Guangzhou, China) for providing study medications, and the patients who took consent in participation in our study.
Funding: Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme 2017 (to Dr. Guan); Changjiang Scholars and Innovative Research Team in University ITR0961, The National Key Technology R&D Program of the 12th National 5-year Development Plan 2012BAI05B01 and National Key Scientific & Technology Support Program: Collaborative innovation of Clinical Research for chronic obstructive pulmonary disease and lung cancer No. 2013BAI09B09 (to Prof. Zhong).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Protocol approval was obtained from Ethics Committee of participating sites and China Food and Drug Administration [No.: 2009L03443; Medical Ethics Year 2009 (the 12th) and Medical Ethics Year 2016 (the 33rd) for follow-up study]. Informed consent forms were signed before enrollment.
References
- Maziak DE, Todd TR, Keshavjee SH, et al. Adenoid cystic carcinoma of the airway: thirty-two-year experience. J Thorac Cardiovasc Surg 1996;112:1522-31. [Crossref] [PubMed]
- Kokturk N, Demircan S, Kurul C, et al. Tracheal adenoid cystic carcinoma masquerading asthma: a case report. BMC Pulm Med 2004;4:10. [Crossref] [PubMed]
- Gaissert HA, Grillo HC, Shadmehr MB, et al. Long-term survival after resection of primary adenoid cystic and squamous cell carcinoma of the trachea and carina. Ann Thorac Surg 2004;78:1889-96. [Crossref] [PubMed]
- Gao Y, Gao Y, Guan W, et al. Antitumor effect of para-toluenesulfonamide against lung cancer xenograft in a mouse model. J Thorac Dis 2013;5:472-83. [PubMed]
- Li SY, Li Q, Guan WJ, et al. Effects of para-toluenesulfonamide intratumoral injection on non-small cell lung carcinoma with severe central airway obstruction: A multi-center, non-randomized, single-arm, open-label trial. Lung Cancer 2016;98:43-50. [Crossref] [PubMed]
- Osborn DA. Morphology and the natural history of cribriform adenocarcinoma (adenoid cystic carcinoma). J Clin Pathol 1977;30:195-205. [Crossref] [PubMed]
- Velez Jo ET, Morehead RS. Hemoptysis and dyspnea in a 67-year-old man with a normal chest radiograph. Chest 1999;116:803-7. [Crossref] [PubMed]
- Soldà C, Pastorelli D, Bridda A. Tracheal Cacner: A comprehensive Review. Ann Otolaryngol Rhinol 2015;3:1079.
- Lee JH, Jung EJ, Jeon K, et al. Treatment outcomes of patients with adenoid cystic carcinoma of the airway. Lung Cancer 2011;72:244-9. [Crossref] [PubMed]
- Eom JS, Kim B, Kim H, et al. Fibrotic airway stenosis following radiotherapy in patients with adenoid cystic carcinoma. Respirology 2014;19:914-20. [Crossref] [PubMed]
- Shimizu J, Oda M, Matsumoto I, et al. Clinicopathological study of surgically treated cases of tracheobronchial adenoid cystic carcinoma. Gen Thorac Cardiovasc Surg 2010;58:82-6. [Crossref] [PubMed]