Systemic blood pressure trends and antihypertensive utilization following continuous-flow left ventricular assist device implantation: an analysis of the interagency registry for mechanically assisted circulatory support
Introduction
Continuous-flow left ventricular assist devices (CF-LVADs) have emerged as a standard treatment option for patients with refractory, inotrope-dependent heart failure, with 1- and 2-year actuarial survival rates of 80% and 70%, respectively, for the current generation of Food and Drug Administration (FDA)-approved devices (1). Since the output from CF pumps is afterload dependent (2,3), systemic blood pressure (SBP) is believed to be inextricably linked to the performance of these LVADs and hence, to the clinical outcomes of the patients in which they are implanted (3). It has therefore been postulated that systemic hypertension can contribute to the risk of aortic insufficiency, device thrombosis, stroke, and even death in patients with CF devices (3-6).
However, there is little evidence in the literature to support such suppositions as our community has yet to rigorously characterize the impacts that systemic hypertension can have on patients with these pumps. More fundamentally, largely missing from our evidence base is a basic demonstration of how SBP changes after CF-LVAD implantation and further lacking are descriptions of how hypertension is actually being treated when encountered in this mode of mechanical circulatory support (MCS).
We therefore queried the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) in order to more clearly describe the response of SBP to CF-LVAD implantation and to delineate contemporary trends in antihypertensive (AH) management for patients with these life-sustaining devices.
Methods
Data source
INTERMACS was established in 2005 as a joint effort of the National Heart, Lung, and Blood Institute, the FDA, clinicians, scientists, and industry representatives; and is housed and maintained by the University of Alabama at Birmingham (7). This prospective registry collects clinical data on heart failure patients receiving MCS and is recognized as the Joint-Commission-mandated registry for all US centers implanting devices for destination therapy (DT) (7). The registry captures: pre-implant patient demographics and characteristics, in-hospital and procedural data related to MCS device implantations, as well as post-implant follow-up information, including adverse events and rehospitalizations (7). INTERMACS has a Data and Clinical Coordinating Center (DCC) comprised of experts who check the data from each site for internal validity remotely. Sites with suspicious data or data discrepancies are flagged and audited. To date, INTERMACS contains records on upwards of 10,000 MCS device implantations, contributed by over 200 participating hospitals (1), making it the largest repository of contemporary data on MCS patients in the world. The registry has been used during device approval, in post-approval follow-up studies, and as a vital resource in examining many specific MCS-related questions (8-10).
This study conforms to the policies set forth by both the INTERMACS Data Access, Analysis, and Publications Committee and the INTERMACS Data Coordinating Center and was exempted from review by the Institutional Review Board of Weill Cornell Medical College since registry data are de-identified and stored in aggregate. This project was funded, in whole or in part, with federal funds from the National Heart, Lung, and Blood Institute of the National Institutes of Health, Department of Health and Human Services, under contract number HHSN268201100025C.
Patient population and data collection
We queried INTERMACS to identify all adult (i.e., age greater than 18 years) CF-LVAD implantations from 2006 to 2014, excluding those whose durations were less than 30 days. Pre-implant patient demographics and characteristics were obtained for each of these implantation records, which included: age, gender, body mass index, primary cardiac diagnosis, comorbidities, INTERMACS profile, SBP, echocardiographic data, device strategy, and AH history. Pre-implant data is collected during the index hospitalization for implantation. Specifically, this data is collected at the time of implant or closest to the implant date and within 30 days of implant, but is not collected in the operating room.
SBPs, AH-utilization data, and vital status were then obtained for each of the implant records identified for inclusion, beginning at 1-week post-implant and extending up to 5 years after device implantation. It is important to note that AH-utilization prior to LVAD implantation was a representation of neurohumoral modulatory agent utilization as patients at this point were likely to be receiving therapy for heart failure and not exclusively for hypertension. Of note, SBPs are recorded in INTERMACS as either systolic/diastolic pressures or as single Doppler opening pressures. Herein, we express all systemic pressures as mean arterial pressures (MAPs), which were calculated using the systolic/diastolic pressures when only those measurements were available. If both systolic/diastolic and Doppler values were documented or when only Doppler pressures were recorded, the MAPs were assumed to be equal to the Doppler pressures and were tabulated as such.
Study objectives and statistical analyses
The objectives of this study were two-fold: (I) to characterize trends of the response of SBP to CF-LVAD implantation on a large scale and (II) to describe the trends in AH utilization following CF-LVAD implantation, examining for possible changes in medication usage by implant era.
Patient demographics and characteristics as well as follow-up blood pressures and AH-use data were expressed as frequencies and percentages for categorical variables and as means ± standard deviations (SDs) for continuous variables. Categorical variables were compared using the Pearson chi-square test; all P values were two-sided with statistical significance evaluated at the 0.05 α level. No missing-values estimations were performed, as only data that were available at a given time point were reported. Statistical analyses were performed using SPSS Version 19 (IBM Corp., Armonk, NY, USA) and Excel 2013 (Microsoft Corp., Redmond, WA, USA).
Results
Patient demographics and characteristics
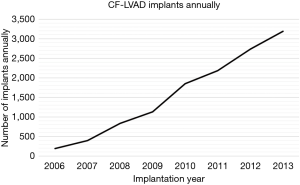
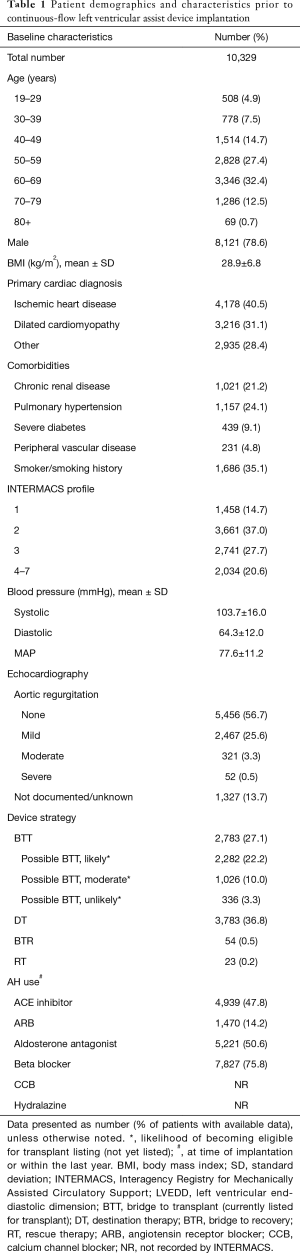
Over the course of the study period, the rate of CF-LVAD implantation increased annually (Figure 1). A total of 10,329 CF-LVAD implantations were included for study. Table 1 summarizes the demographics and pre-implantation characteristics of the patients representing this cohort. A plurality of implantations (32.4%, n=3,346) occurred during the seventh decade of life and most were in males (78.6%, n=8,121). Ischemic heart disease was the most common primary cardiac diagnosis (40.5%, n=4,178) within the cohort; 35.1% (n=1,686) of implantations were in patients with smoking histories, while upwards of 20% were in patients with chronic renal disease and/or pulmonary hypertension (21.2%, n=1,021; 24.1%, n=1,157, respectively). The mean MAP prior to CF-LVAD implantation was 77.6±11.2 mmHg; on average, patients required 1.9±1.0 AHs. Three-quarters of implantations occurred in patients on beta blockers (75.8%, n=7,827) and nearly half occurred in those on aldosterone antagonists (50.6%, n=5,221) and/or angiotensin-converting-enzyme (ACE) inhibitors (47.8%, n=4,939).
Full table
SBP response to CF-LVAD implantation
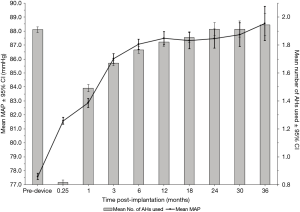
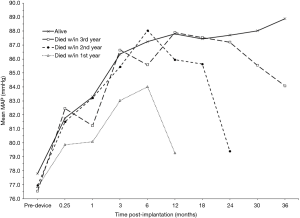
Figure 2 depicts the response of SBP to CF-LVAD implantation (black line, left axis). SBPs increased rapidly over the first three post-implant months but plateaued thereafter; by 6 months, the mean MAP had increased to 87.1 mmHg (95% CI: 86.7–87.4), a 12.2%-increase from pre-implantation (77.6 mmHg, 95% CI: 77.4–77.8). Stratifying this blood pressure response by annual survival revealed that systemic pressures, after reaching a plateau, fell precipitously within the year preceding death (Figure 3).
AH utilization following CF-LVAD implantation
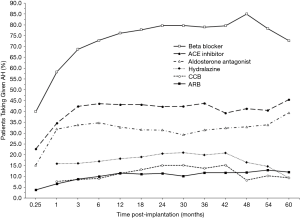
Overall AH requirements increased after device implantation, as also illustrated by Figure 2 (grey bars, right axis). Similar to the trend seen for SBP response, the mean number of AHs used quickly rose following implantation but leveled-off after 3 months (Figure 2). At 6 months, patients required a mean of 1.8 (95% CI: 1.75–1.78) AH medications, which represents a 125% increase from AH use at 1-week post-implantation (0.8 AHs/patient, 95% CI: 0.81–0.83) but a 5.3% decrease from pre-implant utilization (1.9 AHs/patient, 95% CI: 1.90–1.92). By 2 years, the mean AH requirement (1.9 AHs/patient, 95% CI: 1.89–1.94) was unchanged from the pre-implantation baseline. After CF-LVAD implantation, beta blockers were the most frequently used AHs, followed by ACE inhibitors and aldosterone antagonists (Figure 4). Beta-blocker, ACE-inhibitor, and aldosterone-antagonist utilizations all appeared to increase rapidly in the first 3 months post-implant (Figure 4).
The most common AH regimens employed at 1 week, 1 month, and 3 months of follow-up are shown in Figure 5. This analysis was limited to these early time points as most of the changes in SBPs and AH utilization occurred with the first 3 months after CF-LVAD implantation; 44% (n=4,463) of patients required no AHs and 21% (n=2,102) were on sole beta blockade at 1 week; by 3 months, only 10% (n=484) of patients remained off AHs. By that point, a plurality of patients were on lone beta-blocker therapy (15%, n=720); the next most common regimens at 3 months post-implantation were the combination of a beta blocker and an ACE inhibitor (14%, n=672) followed by triple-agent therapy with a beta blocker, an ACE inhibitor, and an aldosterone antagonist (11%, n=540) (Figure 5).

We additionally examined whether implant era played any role in AH management, choosing again to focus only on early time points (i.e., 1 and 3 months post-implant) given that these represented the period of greatest flux in both SBPs and medication use (Figure 6). For patients whose devices were implanted in 2012, the 1-month frequencies-of-use of beta blockers, aldosterone antagonists, and angiotensin receptor blockers (ARBs) were all significantly higher than their respective values in the baseline year of 2008 (62.4% vs. 50.5%, P<0.001; 35.6% vs. 25.6%, P=0.003; and 7.1% vs. 3.6%, P=0.050; respectively). The 1-month frequencies-of-use of beta blockers and ARBs were also significantly higher in 2013, compared again to their respective baseline values in 2008 (58.4% vs. 50.5%, P=0.022 and 7.5% vs. 3.6%, P=0.032, respectively) (Figure 6, top panel).

The frequencies-of-use of beta blockers and aldosterone antagonists were significantly higher in subsequent years at the 3-month time point as well: beta blockers 71.8% in 2011 (P=0.009) and 71.3% in 2012 (P=0.014) vs. 65.3% in 2008 and aldosterone antagonists 31.1% in 2010 (P=0.007), 33.7% in 2011 (P<0.001), 36.9% in 2012 (P<0.001), and 36.4% in 2013 (P<0.001) vs. 24.1% in 2008 (Figure 6, bottom panel).
Discussion
This study constitutes the most comprehensive examination of SBP and AH management in patients supported with CF-LVADs. In leveraging the INTERMACS registry, we were able to describe blood pressure and medication trends representative of the “real-world” experience with this type of device, demonstrating that: (I) systemic pressures rose quickly following pump implantation and, among long-term survivors, stabilized after 3 to 6 months; (II) these early changes in blood pressure were matched by concordant changes in AH use; and (III) AH utilization increased with chronologic year of implantation.
In their analysis of blood pressure control in 96 CF-LVAD patients, Lampert et al. similarly demonstrated that MAPs climbed steadily in response to device implantation and that these increases occurred early (within 3 months) during the post-implant course (5). Although the authors followed a predefined institutional blood pressure protocol that set a goal MAP of less than 80 mmHg, their cohort’s mean MAP was in excess of this as early as 2 months post-implantation (5). By 4 months, for example, patients’ MAPs averaged near 85 mmHg (5). We found that systemic pressures ascended rapidly to rather high levels in our study population as well. While we do not know the goal pressures the physicians of the patients in our cohort were targeting—for reference, the 2013 International Society for Heart and Lung Transplantation (ISHLT) MCS guidelines recommend a goal MAP of ≤ 80 mmHg (11)—it is obvious that patient MAPs were well above what most in the field would judge as optimal. Moreover, we cannot definitively say whether the observed high mean MAPs in the present study were a result of lax treatment or were due to pressures that proved intransigent in the face of appropriate AH therapy.
The observation that AH utilization was increasing synchronously with increasing systemic pressures suggests that clinicians, at least partially, were attempting to bring MAPs under control. However, considering that at 6 months post-implantation patients’ MAPs were over 10% higher than pre-implant, one might expect that patients would have been on a resultant greater number of AHs as well; yet, we observed that patients were actually on fewer AH medications at that time point compared to pre-implantation. Additionally, at 2 years, when patients were finally on the same number of medications as they had been prior to device implantation, their MAPs were on average almost 13% higher than what they had been pre-device—this suggests that responsiveness to AH medications may be reduced in patients following CF-LVAD implantation. It is noteworthy that almost half of our cohort was INTERMACS 1–2 before LVAD implantation, meaning that some of these patients were on AHs for neurohumoral modulation of heart failure and not specifically for hypertension; however, with that being said, this does not change the observation the AH utilization continued to synchronously increase with increasing systemic pressures after LVAD implantation.
The AH agents and regimens used in managing this hypertension also deserve closer attention. The immediate 3-month period following CF-LVAD implantation was notable for both the observed rapid expansion in overall AH use and the quick pace with which the frequencies-of-use of the individual drugs were growing. Naturally, these significant early changes in how often certain drugs were being used meant that the composition of AH regimens also changed markedly during this time period (Figure 5). Notably, we did not include an analysis of diuretics in this cohort; as the ISHLT only recommends diuretics (class IC) if post LVAD implantation patients in the case of volume overload and ventricular dysfunction, we chose to exclude diuretics in order to avoid further confounding our results that aim to focus on blood pressure control. Since there are no guidelines that outline which AHs should be used as first-line or adjunctive agents in CF-LVAD patients, one can only speculate as to the motivations underlying this shifting makeup. Ideally, our community would determine which AH regimens are most efficacious in these patients but this would likely prove statistically challenging in the presence of so many confounding time-dependent covariates.
Among the reasons for INTERMACS being such a powerful resource is the fact that the registry has captured data on almost all CF-LVAD implantations in the US over nearly the past decade. Coupling this strength with the well-publicized (12) finding that a recent unexpected uptick in CF-device thromboses was implant-era dependent led us to examine what influence, if any, year of implantation had on AH utilization (Figure 6). We noticed that the 1- and 3-month frequencies-of-use of three AH agents increased significantly over time compared to the baseline year of 2008. Most strikingly, 1-month beta-blocker utilization had grown nearly 25% by 2012, while 1-month aldosterone-antagonist use and ARB use had increased 39% and 97% by that year, respectively. It was around this same time—2011/2012—that the incidence in pump thrombosis was noted to be rising (12). However, what effects these era-dependent increases in AH utilization may have had on outcomes was not assessed in the present context and this therefore warrants investigation.
Our intention in this descriptive project was to provide analyses that were both clinically relevant and widely applicable; it is with this in mind that we elected to study SBPs in terms of MAPs, regardless of how the pressures were obtained (e.g., by automated cuff, traditional sphygmomanometry and auscultation, or by Doppler). In so doing, we treated the Doppler opening pressure as representing the MAP in those patients for whom this measurement was available. Doppler pressures have been shown to correlate well with MAPs obtained via arterial catheters, more accurately reflecting the MAP than the systolic blood pressure in patients with CF pumps (13). Although Doppler pressures may sometimes overestimate true MAPs in cases of preserved pulsatility (14), considering that the aforementioned ISHLT guidelines designate ideal MAPs (as opposed to goal systolic/diastolic pressures), we felt that using MAPs in the present study would be more clinically relatable.
Although ours is the largest study yet delving into the complex issue of systemic hypertension in patients with CF-LVADs, this work does have several limitations. First, INTERMACS does not disclose the subtype (i.e., axial-flow versus centrifugal) of the pumps that are implanted; as such, we were unable to distinguish between the two in our cohort and therefore cannot comment on what effect(s) differences in pump design may have had on SBPs. In their analysis, though, Lampert et al. found no statistically significant differences in mean MAPs among those patients implanted with axial-flow versus centrifugal devices over the first 5 months of support (5). Second, INTERMACS does not collect detailed information with respect to the exact agents prescribed within a given class of AHs nor does the registry capture dosing data; we were thereby further limited in our descriptive abilities. Third, anti-hypertensive agents such as beta-blockers, aldosterone antagonists and nitrates can be used for purposes other than blood pressure control, which may have confounded our results. Finally, as most MCS specialists would agree, what is ultimately important for our community to figure out is determining the impact that post-implant systemic pressures have on specific outcomes like aortic insufficiency, pump thrombosis, stroke, and survival. As alluded to earlier, the kind of longitudinal study that could potentially do this using the current data would be inherently complex in that it would involve numerous intertwined time-dependent covariates. Such covariates, of which blood pressure is one, vary with time and (I) can influence other measured variables (which themselves may change over time, like aortic regurgitation), and (II) may change in response to one or more of these other measured variables (15). Hence, in the present study, it was not our goal to answer the question: “how does systemic hypertension at any point in time after CF-LVAD implantation affect outcome X?”
Conclusions
SBP rises rapidly following CF-LVAD implantation, often in excess of currently accepted norms, resulting in concomitant increases in AH use—use that has grown over consecutive implant years. Despite this ever-increasing utilization, responsiveness to AH medications may be reduced in patients following CF-LVAD implantation. The descriptions provided herein should catalyze research on the role of SBP in determining clinical outcomes for patients with CF pumps.
Acknowledgements
Funding: This project was funded, in whole or in part, with federal funds from the National Heart, Lung, and Blood Institute of the National Institutes of Health, Department of Health and Human Services (HHSN268201100025C).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant 2014;33:555-64. [Crossref] [PubMed]
- Griffith BP, Kormos RL, Borovetz HS, et al. HeartMate II left ventricular assist system: from concept to first clinical use. Ann Thorac Surg 2001;71:S116-20. [Crossref] [PubMed]
- Wasson LT, Yuzefpolskaya M, Wakabayashi M, et al. Hypertension: an unstudied potential risk factor for adverse outcomes during continuous flow ventricular assist device support. Heart Fail Rev 2015;20:317-22. [Crossref] [PubMed]
- Najjar SS, Slaughter MS, Pagani FD, et al. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant 2014;33:23-34. [Crossref] [PubMed]
- Lampert BC, Eckert C, Weaver S, et al. Blood pressure control in continuous flow left ventricular assist devices: efficacy and impact on adverse events. Ann Thorac Surg 2014;97:139-46. [Crossref] [PubMed]
- Nassif ME, Tibrewala A, Raymer DS, et al. Systolic blood pressure on discharge after left ventricular assist device insertion is associated with subsequent stroke. J Heart Lung Transplant 2015;34:503-8. [Crossref] [PubMed]
- Interagency Registry for Mechanically Assisted Circulatory Support. Available online: http://www.uab.edu/medicine/intermacs/
- Aaronson KD, Slaughter MS, Miller LW, et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 2012;125:3191-200. [Crossref] [PubMed]
- Jorde UP, Kushwaha SS, Tatooles AJ, et al. Results of the destination therapy post-food and drug administration approval study with a continuous flow left ventricular assist device: a prospective study using the INTERMACS registry (Interagency Registry for Mechanically Assisted Circulatory Support). J Am Coll Cardiol 2014;63:1751-7. [Crossref] [PubMed]
- Grady KL, Naftel DC, Myers S, et al. Change in health-related quality of life from before to after destination therapy mechanical circulatory support is similar for older and younger patients: Analyses from INTERMACS. J Heart Lung Transplant 2015;34:213-21. [Crossref] [PubMed]
- Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant 2013;32:157-87. [Crossref] [PubMed]
- Mehra MR, Stewart GC, Uber PA. The vexing problem of thrombosis in long-term mechanical circulatory support. J Heart Lung Transplant 2014;33:1-11. [Crossref] [PubMed]
- Bennett MK, Roberts CA, Dordunoo D, et al. Ideal methodology to assess systemic blood pressure in patients with continuous-flow left ventricular assist devices. J Heart Lung Transplant 2010;29:593-4. [Crossref] [PubMed]
- Lanier GM, Orlanes K, Hayashi Y, et al. Validity and reliability of a novel slow cuff-deflation system for noninvasive blood pressure monitoring in patients with continuous-flow left ventricular assist device. Circ Heart Fail 2013;6:1005-12. [Crossref] [PubMed]
- Daniel RM, Cousens SN, De Stavola BL, et al. Methods for dealing with time-dependent confounding. Stat Med 2013;32:1584-618. [Crossref] [PubMed]


